Chapter: Modern Analytical Chemistry: Kinetic Methods of Analysis
Radiochemical Methods of Analysis: Evaluation
Evaluation
Radiochemical methods are routinely used for
the analysis of trace analytes in macro- and
mesosamples. The accuracy and precision of radiochemical analyses are generally within the range of 1–5%. Precision
is limited by the random nature of ra-
dioactive decay and is improved by counting the emission of radioactive
particles over as long a time as is practical. If the number
of counts, M, is
reasonably large (M >= 100),
and the counting
period is significantly less than the isotopes half-life, then the percent relative
standard deviation for the activity, (σA)rel, is estimated as
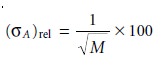
For example, when the activity
is determined by counting 10,000
radioactive parti- cles, the
relative standard deviation is 1%. The
analytical sensitivity of a radiochemi- cal method is inversely
proportional to the standard deviation
of the measured ac- tivity and,
therefore, is improved by increasing the
number of particles that are counted.
Selectivity rarely is of concern
with radiochemical methods
because most sam- ples contain only a single radioactive isotope. When several
radioactive isotopes are present, differences in the energies of their respective radioactive particles can be
used to determine each isotope’s activity.
In comparison with most other analytical techniques, radiochemical methods
are usually more expensive and require more time to complete an analysis. Radio- chemical methods also are subject to significant safety
concerns due to the analyst’s potential exposure to high-energy radiation and the need
to safely dispose
of ra- dioactive waste.
Related Topics