Chapter: Health Management in Aquaculture: Parasitic diseases and pests
Protozoan Infestations: Ciliates, Flagellates, Myxosporeans - Fish Diseases Caused by Parasites
Protozoan Infestations
Protozoans are unicellular, microscopic organisms with specialized structuresfor locomotion, food gathering, attachment, and protection. They can multiply on or within their hosts.
Ciliates have short, fine cytoplasmic outgrowths called cilia as the locomotoryorganelle. They are either attached or motile. Ciliates are mainly ectoparasitic.
CAUSATIVE AGENTS:
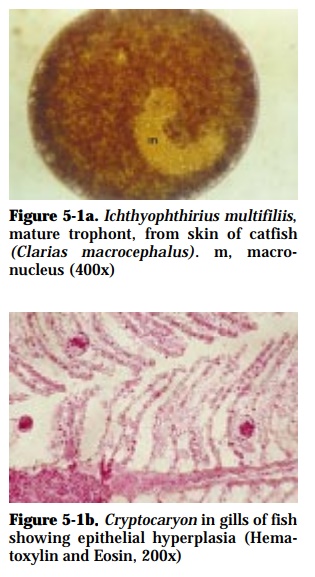
Ichthyophthirius multifiliis (50-1000 µm diameter) in freshwater (Fig. 5-1a)
Cryptocaryon irritans (60-450 µm diameter) in marine and brackishwater
The disease is known as Ichthyophthiriasis (“Ich”) or White Spot Disease
SPECIES AFFECTED:
Catfish, carp, tilapia, seabass, grouper, snapper
GROSS SIGNS:
The disease is called “white spot” because of the presence of a few to nu-merous whitish or grayish spots on the skin and gills of affected fish which are actually nests of these parasites. Diseased fish lose their appetite, are lethargic, with dull, opaque or hemorrhagic eyes. Heavily infested fish pro-duce a lot of mucus and they rub their body against the substrate or sides of tanks.
EFFECTS ON HOST:
This disease causes severe epizootic especially in intensive culture systems. The parasite may destroy the skin and gills (Fig. 5-1b). Ulcers may develop in the skin of heavily infested fish and may be the sites of secondary bacte-rial or fungal infection. Occurrence of this parasite is usually associated with a drop in temperature to 28°C.
DIAGNOSIS:
Encysted (0.10-0.35 mm) organisms appear as white spots on the surface of fish and can be seen by the naked eye. Microscopic examination of mucus from the body surface and gill filaments reveals round or oval parasites, propelled by cilia and possessing a horseshoe-shaped macronucleus in the case of Ichthyophthirius.
PREVENTION AND CONTROL:
For “Ich”
• Increase water temperature to 30°C for 6 h daily for 3-5 d
• 0.05% salt solution
• 100 ppm formalin for 1 h for 2-3 d
• 25 ppm formalin + 0.1 ppm malachite green
• Transfer infected stock in dry, parasite-free tanks for 2-3 times at 3 d interval
For Cryptocaryon
• 0.5 ppm CuSO4 and 25 ppm formalin for 5-7 d, then transfer to dry, para-site-free tanks for 2 times at 3 d interval.
CAUSATIVE AGENTS:
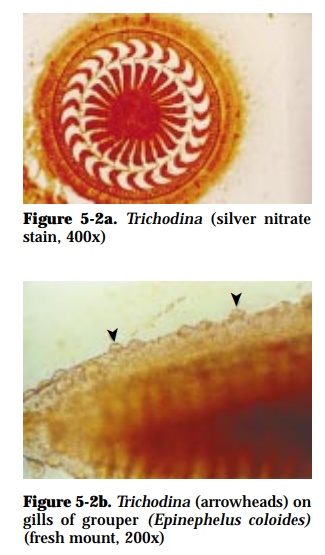
Trichodina (45-78 µm diameter) (Fig. 5-2a), Trichodinella (24-37 µmdiameter), Tripartiella (up to 40 µm diameter)
SPECIES AFFECTED:
Carp, tilapia, milkfish, seabass, mullet, siganid, grouper, snapper
GROSS SIGNS:
The parasites are attached mainly on the gills (Fig. 5-2b) and skin of the host. Affected fish appear weak with excessive mucus production and with frayed fins.
EFFECTS ON HOST:
Excessive numbers of the parasite on the skin and gills of infested fish may interfere with respiration. High mortality was observed among young fish. The adhesive disc can cause direct damage to the branchial epithelium re-sulting in gill lesions.
DIAGNOSIS:
Microscopic examination of wet mounts of gill filaments and scrapings from skin show saucer-shaped organisms, surrounded by cilia around its perim-eter.
PREVENTION AND CONTROL:
• 2-3% salt solution for 2-5 min for 3-4 d (carp fry)
• 100% freshwater bath for 1 h for 3 d
• 100 ppm formalin+10 ppm Acriflavin for 1 h for 3 d
CAUSATIVE AGENT:
Brooklynella (36-86 x 32-50 µm)
SPECIES AFFECTED:
Grouper, seabass, snapper
GROSS SIGNS:
The parasite attaches to the skin and gills of fish. Affected fish rub body against objects causing extensive skin damage and subcutaneous hemor-rhage.
EFFECTS ON HOST:
May result to respiratory difficulties; may develop secondary bacterial infec-tion
DIAGNOSIS:
Microscopic examination of mucus from body surface of affected fish and gill filaments show bean-shaped protozoans with long parallel lines of cilia that beat in waves.
PREVENTION AND CONTROL:
• 100% freshwater bath for 1 h for 3 days
• 100 ppm formalin for 1 h for 2-3 days
Flagellates have one or more long, hair-like structures called flagella used as alocomotory organelle. They occur on the skin, gills, intestinal organs, and blood of fish.CAUSATIVE AGENT:
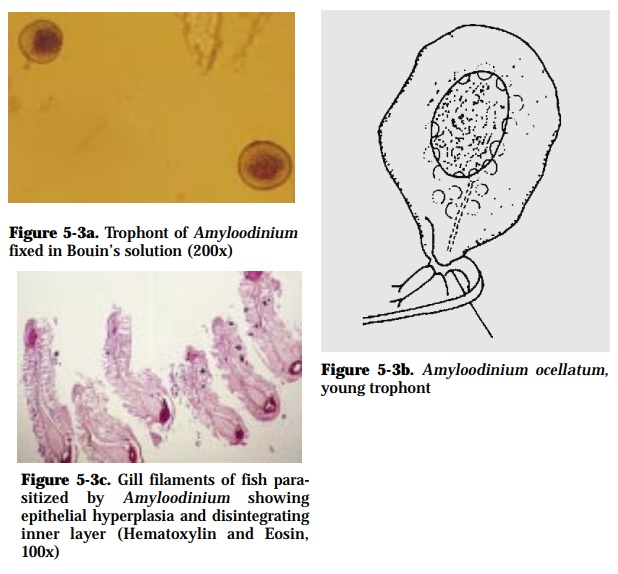
Amyloodinium ocellatum (150-350 x 15-70 µm) (Fig. 5-3a, 5-3b)
SPECIES AFFECTED:
Mullet, siganid, grouper
GROSS SIGNS:
Heavily infested skin may have a dusty appearance (‘velvet disease’) with excessive mucus production. The parasite also attaches to the gills of af-fected fish. Fish rub body against objects in tanks. Affected fish exhibit ab-normal surface swimming (spasmodic gasping and uncoordinated move-ments).
EFFECTS ON HOST:
This disease has been reported to cause morbidity and mortality in marine and brackishwater fishes. Heavy infestation can cause death within half a day. Histopathological changes include disintegration of the affected tissues (Fig. 5-3c), severe gill epithelial hyperplasia and reduced or absence of mu-cus cell.
DIAGNOSIS:
Microscopic examination of gill filaments or skin scrapings will reveal pear or ovoid-shaped trophonts with elongated red stigma near attachment site.
PREVENTION AND CONTROL:
• Use of sand filters; ultraviolet irradiation of rearing water
• Disinfection of culture facilities using lime
• Quarantine of new stocks
• Freshwater bath can cause parasite to drop off the gills
• 0.75 ppm CuSO4 for 5-6 days
• 25 ppm formalin plus 0.1 ppm malachite green for 1 day
• 100-300 ppm formalin, 10 min
CAUSATIVE AGENTS:
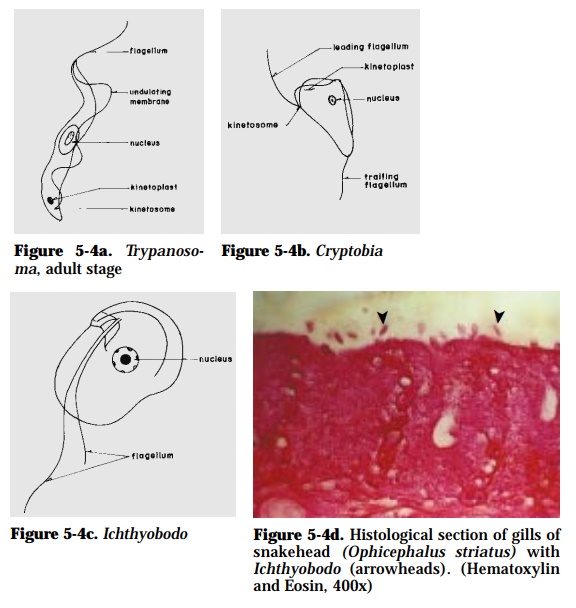
Trypanosoma (18-32 µm) (Fig. 5-4a), Cryptobia (15 µm) (Fig. 5-4b), Ichthyobodo (10-15 µm) (Fig. 5-4c)
SPECIES/TISSUE AFFECTED:
Snakehead, carps, mullet, milkfish (blood)
GROSS SIGNS:
Affected fish have greyish-white film on fins and body surface, with frayed or destroyed fins. Fish rub their body against immersed objects or sides of the tank. Ichthyobodo is attached mainly on dorsal fins and gills (Fig. 5-4d) of the host. Trypanosoma and Cryptobia are parasitic on the blood of fish.
EFFECT ON HOST:
Affected fish show sluggishness, pale gills, and emaciated body. Fish para-sitized by blood protozoans are usually anemic.
DIAGNOSIS:
For Ichthyobodo, microscopic examination of mucus from body surface and gill filaments. For blood protozoans, blood smears fixed in methanol and stained with Giemsa are examined under high power magnification (100x) of a compound microscope.
PREVENTION AND CONTROL:
• Drying of culture facilities
• Use of filters
• Elimination of the vector (leech) for blood protozoans
• Application of 10 ppt, 15-30 min or 2-5 ppm KMnO4
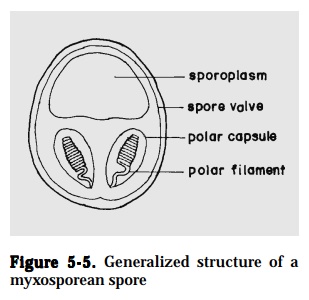
Myxosporeans – the spore (7-20 µm) is the infective stage, and is composed of1 to 7-spore shell valves, 1 to 2-sporoplasms and 2 to 7-polar capsules. Myxosporeans are parasitic in organ cavities and tissues of fish (Fig. 5-5).
CAUSATIVE AGENT:
Myxidium, Myxobolus, Henneguya, Kudoa, Myxosoma, Thelohanellus
SPECIES AFFECTED:
Mullet, catfish, eel, carps, climbing perch, snakehead
GROSS SIGNS:
White cysts are formed on skin, gills, muscle, brain, heart, ovaries, or other internal organs of fish. Myxosporean cysts produce thick milky exudate when ruptured.
EFFECT ON HOST:
Heavy gill infections may lead to respiratory dysfunction. Several cysts formed in the muscle may render the fish unmarketable. Heavy infection in internal organs may result to loss of equilibrium, skeletal deformities, and destruction of the host tissue.
DIAGNOSIS:
Microscopic examination of fresh smears of cysts containing many infective spores.
PREVENTION AND CONTROL:
• Isolate and destroy infected fish
• Disinfect rearing facilities with lime
CAUSATIVE AGENT:
Sphaerospora (8.7 x 8.2 µm; with 2 spherical polar capsules)
SPECIES AFFECTED:
Grouper, seabass, marine catfish
GROSS SIGNS:
Affected fish exhibit swollen abdomen, exophthalmia and anemia.
EFFECTS ON HOST:
Spore stages are found in kidney, liver, gall bladder, and blood cells. In-fected kidney tubules display severe vacuolation of the epithelium.
DIAGNOSIS:
Microscopic examination of fresh preparations of kidney and blood smears stained with Giemsa.
PREVENTION AND CONTROL:
Ultraviolet treatment of inflow water can control the infective stage, but is usually impractical
Related Topics