Chapter: Medical Physiology: Somatic Sensations: II. Pain, Headache, and Thermal Sensations
Pain Suppression (ÔÇťAnalgesiaÔÇŁ) System in the Brain and Spinal Cord
Pain Suppression (ÔÇťAnalgesiaÔÇŁ) System in the Brain and Spinal Cord
The degree to which a person reacts to pain varies tremendously. This results partly from a capability of the brain itself to suppress input of pain signals to the nervous system by activating a pain control system, called an analgesia system.
The analgesia system is shown in Figure 48ÔÇô4. It consists of three major components: (1) The periaque-ductal gray and periventricular areas of the mesen-cephalon and upper pons surround the aqueduct of Sylvius and portions of the third and fourth ventricles. Neurons from these areas send signals to (2) the raphemagnus nucleus, a thin midline nucleus located inthe lower pons and upper medulla, and the nucleusreticularis paragigantocellularis, located laterally inthe medulla. From these nuclei, second-order signals are transmitted down the dorsolateral columns in the spinal cord to (3) a pain inhibitory complex located inthe dorsal horns of the spinal cord. At this point, theanalgesia signals can block the pain before it is relayed to the brain.
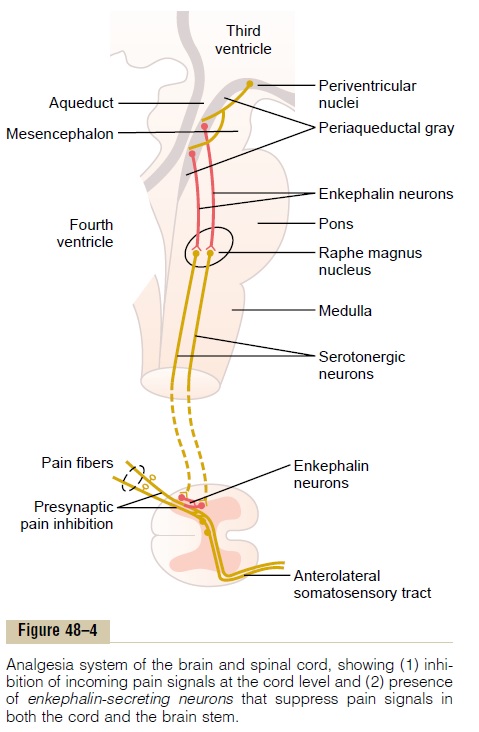
Electrical stimulation either in the periaqueductal gray area or in the raphe magnus nucleus can suppress many strong pain signals entering by way of the dorsal spinal roots. Also, stimulation of areas at still higher levels of the brain that excite the periaqueductal gray area can also suppress pain. Some of these areas are (1) theperiventricular nuclei in the hypothalamus, lying adjacent to the third ventricle, and (2) to a lesser extent, the medial forebrain bundle, also in the hypo-thalamus.
Several transmitter substances are involved in the analgesia system; especially involved are enkephalin and serotonin. Many nerve fibers derived from the periventricular nuclei and from the periaqueductal gray area secrete enkephalin at their endings. Thus, as shown in Figure 48ÔÇô4, the endings of many fibers in the raphe magnus nucleus release enkephalin when stimulated.
Fibers originating in this area send signals to the dorsal horns of the spinal cord to secrete serotonin at their endings. The serotonin causes local cord neurons to secrete enkephalin as well. The enkephalin is believed to cause both presynaptic and postsynapticinhibition of incoming type C and type Adpain fiberswhere they synapse in the dorsal horns.
Thus, the analgesia system can block pain signals at the initial entry point to the spinal cord. In fact, it can also block many local cord reflexes that result from pain signals.
BrainÔÇÖs Opiate SystemÔÇöEndorphins and Enkephalins
More than 35 years ago it was discovered that injec-tion of minute quantities of morphine either into the periventricular nucleus around the third ventricle or into the periaqueductal gray area of the brain stem causes an extreme degree of analgesia. In subsequent studies, it has been found that morphine-like agents, mainly the opiates, also act at many other points in the analgesia system, including the dorsal horns of the spinal cord. Because most drugs that alter excitability of neurons do so by acting on synaptic receptors, it was assumed that the ÔÇťmorphine receptorsÔÇŁ of the analge-sia system must be receptors for some morphine-like neurotransmitter that is naturally secreted in the brain. Therefore, an extensive search was undertaken for the natural opiate of the brain. About a dozen such opiate-like substances have now been found at differ-ent points of the nervous system; all are breakdown products of three large protein molecules: pro-opiomelanocortin, proenkephalin, and prodynorphin.
Among the more important of these opiate-like substances are b-endorphin, met-enkephalin, leu-enkephalin, and dynorphin.
The two enkephalins are found in the brain stem and spinal cord, in the portions of the analgesia system described earlier, and b-endorphin is present in both the hypothalamus and the pituitary gland. Dynorphin is found mainly in the same areas as the enkephalins, but in much lower quantities.
Thus, although the fine details of the brainÔÇÖs opiate system are not understood, activation of the analgesiasystem by nervous signals entering the periaqueductalgray and periventricular areas, or inactivation of painpathways by morphine-like drugs, can almost totallysuppress many pain signals entering through the peripheral nerves.
Inhibition of Pain Transmission by Simultaneous Tactile Sensory Signals
Another important event in the saga of pain control was the discovery that stimulation of large type A╬▓ sensory fibers from peripheral tactile receptors can depress transmission of pain signals from the same body area. This presumably results from local lateral inhibition in the spinal cord. It explains why such simple maneuvers as rubbing the skin near painful areas is often effective in relieving pain. And it probably also explains why lin-iments are often useful for pain relief.
This mechanism and the simultaneous psychogenic excitation of the central analgesia system are probably also the basis of pain relief by acupuncture.
Treatment of Pain by Electrical Stimulation
Several clinical procedures have been developed for suppressing pain by electrical stimulation. Stimulating electrodes are placed on selected areas of the skin or, on occasion, implanted over the spinal cord, supposedly to stimulate the dorsal sensory columns.
In some patients, electrodes have been placed stereo-taxically in appropriate intralaminar nuclei of the thal-amus or in the periventricular or periaqueductal area of the diencephalon. The patient can then personally control the degree of stimulation. Dramatic relief has been reported in some instances. Also, pain relief has been reported to last for as long as 24 hours after only a few minutes of stimulation.
Related Topics