Chapter: Pathology: Red Blood Cell Pathology: Anemias
Normocytic Anemias
NORMOCYTIC ANEMIAS
Anemias
of blood loss.Acute blood loss may cause shock or death. If the patient
sur-vives, the resulting hemodilution caused by shift of water from the
interstitium will lower the hematocrit. There will be a marked reticulocytosis
in 5–7 days. Chronic blood loss, such as from the gastrointestinal tract or
from the gynecologic system, may result in iron deficiency anemia.
Hemolytic anemias.
·
In intravascular (IV) hemolysis, release of hemoglobin into the blood
causes hemoglobinemia and hemoglobinuria; increased bilirubin from erythrocytes
causes jaundice and an increased risk of pigment (bilirubin) gallstones. The
hemoglobin may be oxidized to methemoglobin, which causes methemoglo-binemia
and methemoglobinuria. Markedly decreased (because they have been used up)
hemoglobin-binding proteins in the blood, such as haptoglobin and hemopexin,
are characteristic. No splenomegaly is seen.
·
In extravascular (EV) hemolysis, splenomegaly results if the EV
hemolysis occurs in the spleen and hepatomegaly results if the EV hemolysis
occurs in the liver. EV hemolyis causes increased bilirubin and decreased
haptoglobin, but not to the degree seen with intravascular hemolysis. In EV
hemolysis, there is an absence of hemoglobinemia, hemoglobinuria, and
methemoglobin formation.
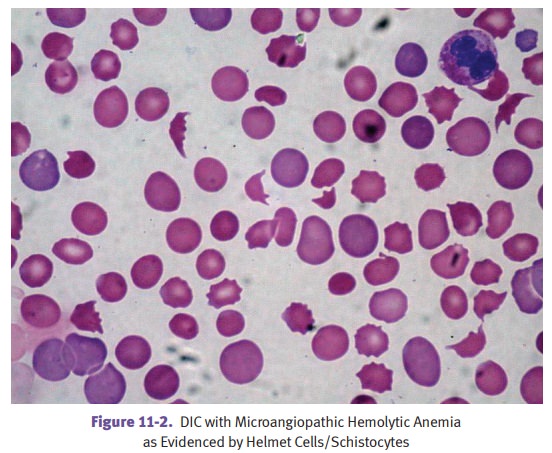
Sickle
cell disease is an inherited blood disorder leading to the formation of
hemo-globin S and increased propensity for the affected red blood cells to
become sickle-shaped and occlude small vessels. The genetic abnormality is a
single nucleotide change that causes valine (neutral) to replace normal
glutamic acid (acidic) at the sixth position of the β-globin
chain. This biochemical change then makes a criti-cal point on the surface of
the hemoglobin molecule become hydrophobic, making it feel “sticky” to an
adjacent hemoglobin molecule, thereby favoring hemoglobin precipitation in
crystalline form.
Heterozygous
(AS) genome causes sickle cell trait. About 8% of African Americans are
heterozygous for hemoglobin S. Patients with sickle trait have fewer symptoms
than those with sickle disease, and also have resistance to Plasmodium falciparum infection (malaria),
which may be why the disease has remained in the human genetic pool. Homozygous
(SS) genome causes clinical disease (sickle cell anemia).
There
are several factors affecting formation of irreversibly sickled red blood
cells.
·
Increased
concentration (dehydration) makes symptoms worse; decreasedconcentration
of sickled hemoglobin (as is seen if a sickle cell patient also has a
thalassemia) makes symptoms better.
·
Decreased
pH decreases oxygen affinity and makes symptoms worse.
·
Increased
hemoglobin F makes symptoms better (rationale for therapy
withhydroxyurea, which increases blood hemoglobin F levels).
·
The presence of hemoglobin C (SC: double-heterozygote individual)
makessymptoms better.
Clinical
features include increased erythrocyte destruction which causes a severe
hemolytic anemia, accompanied by erythroid hyperplasia in the bone marrow and
increased bilirubin leading to jaundice and gallstone (pigment) formation.
Capillary thrombi result from sickle cells blocking small vessels and may cause
vaso-occlusive (painful) crises; hand-foot syndrome (swelling) in children; and
autosplenectomy, which is seen in older children and adults. Howell-Jolly
bodies will appear in periph- eral blood after autosplenectomy, and the lack of
a functional spleen predisposes to increased incidence of infections
(encapsulated organisms), increased incidence of Salmonella osteomyelitis (leg
pain), leg ulcers, and risk of aplastic crisis (especially with parvovirus B19
infection). Emergencies that may occur include priapism and acute chest
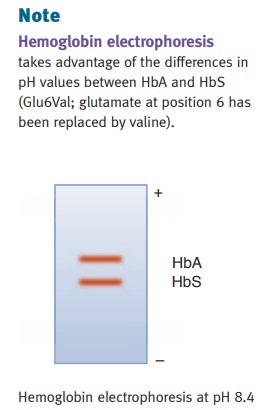
syndrome. For testing, hemoglobin electrophoresis is used to diagnose the
disease, though genetic testing can be performed on amniotic fluid for prenatal
diagnosis. Newborn screening is now mandatory in the United States and is
commonly performed via high performance liquid chromatography. Treatment is
hydroxyurea (to increase hemoglobin F) and hematopoietic stem cell
transplantation.
Hemoglobin
C disease occurs when a single nucleotide change in a codon causes lysine
(basic) to replace normal glutamic acid (acidic) at the beta 6 position. Hemo-
globin C disease is characterized by mild normochromic-normocytic anemia, sple-
nomegaly, target cells, and rod-shaped crystals in erythrocytes (the latter
being characteristic).
Glucose-6-phosphate
dehydrogenase deficiency (G6PD) is a genetic disorder
affect-ing the hexose monophosphate shunt pathway. It results in decreased
levels of the antioxidant glutathione (GSH), leaving erythrocytes sensitive to
injury by oxidant stresses leading to hemolysis. In some variants, G6PD is not
due to decreased synthe-sis but rather to defective protein folding, resulting
in a protein having a decreased half-life. The condition has X-linked
inheritance.
·
In African Americans (A–type) with
G6PD, the hemolysis is secondary to acute oxidative stress, such as oxidative
drugs (primaquine, sulfonamides, anti-tuberculosis drugs), and more typically
by viral or bacterial infections. The hemolysis is intermittent (even if the
offending drug is continued) because only older erythrocytes have decreased
levels of glucose-6-phosphate dehy-drogenase.
·
In individuals with G6PD of Mediterranean type, the disease is
associated with favism due to ingestion of fava beans; more severe hemolysis
occurs because all erythrocytes have decreased glucose-6-phosphate
dehydrogenase activity in that there is both decreased synthesis and decreased
stability.
·
In both forms, the oxidation of hemoglobin forms Heinz bodies; these can-not be seen with normal peripheral blood
stains (Wright-Giemsa) but can be visualized with supravital stains (methylene
blue and crystal violet). The Heinz bodies are “eaten” by splenic macrophages
(extravascular hemolysis), which may form “bite cell” erythrocytes that are
visible on routine peripheral blood smears.
Hereditary spherocytosis (HS)
is an autosomal dominant disorder caused by a defectinvolving ankyrin and
spectrin in the erythrocyte membrane; this causes a decrease in the erythrocyte
surface membrane (spherocytosis). Spherocytes are not flexible and are removed
in the spleen by macrophages (i.e., extravascular hemolysis). This causes
multiple problems, including splenomegaly with a mild to moderate hemolytic
anemia, increased bilirubin and increased risk for jaundice and pigment
gallstones secondary to chronic hemolysis, and increased risk for acute
red-cell aplasia due to parvovirus B19 infection. Laboratory testing shows
increased osmotic fragility and normal MCH with increased MCHC. Treatment is
splenectomy and folic acid.
Autoimmune
hemolytic anemia (AIHA) is most commonly warm AIHA,
in whichthe antibodies are IgG that are usually against Rh antigens and are
active at 37°C. Erythrocytes to which the antibodies attach are removed by
splenic macrophages, which tends to induce splenomegaly as the spleen responds
to the perceived need for increased phagocytosis.
The
etiology varies; most cases are idiopathic, but some cases are related to
auto-immune diseases such as systemic lupus erythematosus, chronic lymphocytic
leu-kemia (CLL), small lymphocytic lymphoma (WDLL), or medications
(penicillin). The peripheral blood smear typically shows microspherocytes, and
laboratory con-firmation can be obtained by demonstrating a positive direct
Coombs test (direct antiglobulin test [DAT]).
Paroxysmal
nocturnal hemoglobinuria (PNH) is a hemolytic anemia caused
by anacquired somatic mutation of a gene (PIGA)
that encodes an anchor for proteins (CD55 and CD59) in the cell membrane,
causing complement-mediated lysis in red cells, white cells, and platelets. The
condition causes episodes (paroxysms) of hemolysis at night.
Acidosis
in vivo occurs during sleep
(breathing slowly retains CO2) and
exercise (lactic acidosis), and the acidosis in turn causes activation of
complement. PNH is a clonal stem cell disorder that affects all blood cell
lines, leading to pancytope-nia (anemia, leukopenia, and thrombocytopenia) that
is apparent in the peripheral blood. Venous thrombosis may ensue.
Most
PNH patients have mild disease and don’t require treatment, but a monoclonal
antibody against complement protein C5 can be therapeutic.
Pyruvate
kinase deficiency is the most common enzyme deficiency
in the glycolyticpathway and involves the enzyme that normally converts
phosphoenolpyruvate to pyruvate. Deficiency leads to decreased ATP with resulting
damage to the erythro-cyte membrane. Clinically, there is a hemolytic anemia
with jaundice from birth.
Hereditary
elliptocytosis is a mild, hereditary, hemolytic anemia caused by a defectin
spectrin. It is characterized by osmotically fragile ovoid erythrocytes
(“ellipto-cytes”).
Aplastic anemia is
the term used when marrow failure causes a pancytopenia ofthe blood. Idiopathic
causes for aplastic anemia are most commonly seen; when the etiology is known,
the aplastic anemia may be due to medications (alkylating agents,
chloramphenicol), chemical agents (benzene, insecticides), infection (EBV, CMV,
parvovirus, hepatitis), or whole body radiation (therapeutic or nuclear
exposure).
Related Topics