Chapter: Genetics and Molecular Biology: An Overview of Cell Structure and Function
Moving Molecules into or out of Cells
Moving Molecules into or out of Cells
Small-molecule
metabolic intermediates must not leak out of cells into the medium. Therefore,
an impermeable membrane surrounds the cytoplasm. To solve the problem of moving
essential small molecules like sugars and ions into the cell, special
transporter protein molecules are inserted into the membranes. These and
auxiliary proteins in the cytoplasm must possess selectivity for the
small-molecules being trans-ported. If the small-molecules are being
concentrated in the cell and not just passively crossing the membrane, then the
proteins must also couple the consumption of metabolic energy from the cell to
the active transport.
The
amount of work consumed in transporting a molecule into a volume against a
concentration gradient may be obtained by consider-ing the simple reaction
where Ao is the
concentration of the molecule outside the cell and Ai is the concentration inside the cell:
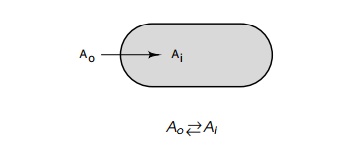
This reaction can be described by an equilibrium
constant
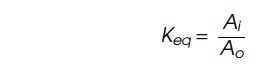
The equilibrium constant Keq, is related to the free energy of the reaction by the relation
ŌłåG=RTlnKeq
where R is about 2 cal/deg.mole and
T is 300┬░ K (about 25┬░ C), the
temperature of many biological reactions. Suppose the energy of hy-drolysis of
ATP to ADP is coupled to this reaction with a 50% efficiency. Then about 3,500
of the total of 7,000 calories available per mole of ATP hydrolyzed under
physiological conditions will be available to the transport system.
Consequently, the equilibrium constant will be
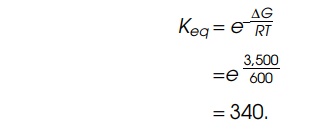
One interesting result of this consideration is
that the work required to transport a molecule is independent of the absolute
concentrations; it depends only on the ratio of the inside and outside
concentrations. The transport systems of cells must recognize the type of
molecule to be transported, since not all types are transported, and convey the
molecule either to the inside or to the outside of the cell. Further, if the
molecule is being concentrated within the cell, the system must tap an energy
source for the process. Owing to the complexities of this process, it is not
surprising that the details of active transport systems are far from being
fully understood.
Four basic types of small-molecule transport
systems have been discovered. The first of these is facilitated diffusion. Here
the molecule
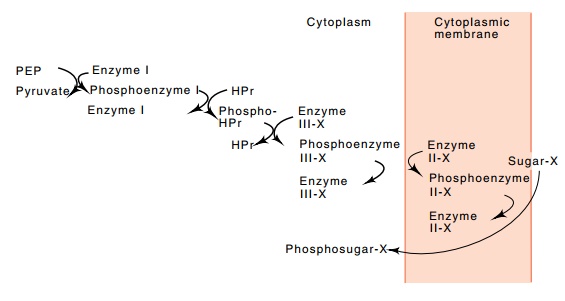
Figure
1.7 The cascade of reactions
associated with the phosphotransferasesugar uptake system of E. coli.
must get into or out of the cell on its own, but
special doors are opened for it. That is, specific carriers exist that bind to
the molecule and shuttle it through the membrane. Glycerol enters most types of
bacteria by this mechanism. Once within the cell the glycerol is phosphorylated
and cannot diffuse back out through the membrane, nor can it exit by using the
glycerol carrier protein that carried the glycerol into the cell.
A second method of concentrating molecules within
cells is similar to the facilitated diffusion and phosphorylation of glycerol.
The phos-photransferase system actively rather than passively carries a number
of types of sugars across the cell membrane and, in the process,
phos-phorylates them (Fig. 1.7). The actual energy for the transport comes from
phosphoenolpyruvate. The phosphate group and part of the chemi-cal
energy contained in the phosphoenolpyruvate is transferred down a series of
proteins, two of which are used by all the sugars transported by this system
and two of which are specific for the particular sugar being transported. The
final protein is located in the membrane and is directly responsible for the
transport and phosphorylation of the trans-ported sugar.
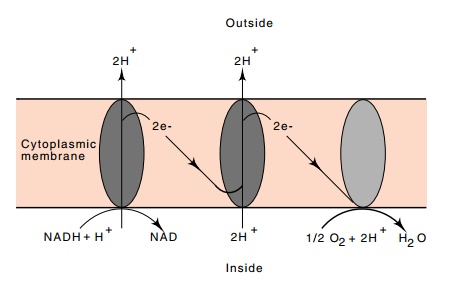
Protons
are expelled from E. coli during the flow of reducing power
from NADH to oxygen. The resulting concentration difference in H+
ions between the interior and exterior of the cell generates a proton motive
force or membrane potential that can then be coupled to ATP synthesis or to the
transport of molecules across the membrane.Active transport systems using this
energy source are called chemiosmotic systems. In the process of permitting a
proton to flow back into the cell, another small molecule can be carried into
the cell, which is called symport, or carried out of the cell, which is called
antiport (Fig. 1.8).
In many
eukaryotic cells, a membrane potential is generated by the sodium-potassium
pump. From the energy of hydrolysis of one ATP molecule, 3 Na+ ions
are transported outside the cell and 2 K+ ions are transported
inside. The resulting gradient in sodium ions can then be coupled to the
transport of other molecules or used to transmit signals along a membrane.
Study of
all transport systems has been difficult because of the necessity of working
with membranes, but the chemiosmotic system has been particularly hard due to
the difficulty of manipulating membrane potentials. Fortunately the existence
of bacterial mutants blocked at
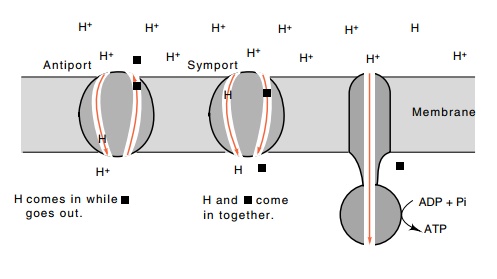
Figure 1.8 Coupling
the excess of H+ions outside a cell to the transport of aspecific
molecule into the cell, symport, or out of the cell, antiport, by specific
proteins that couple the transport of a proton into the cell with the transport
of another molecule. The ATPase generates ATP from ADP with the energy derived
from permitting protons to flow back into the cell.
various steps of the transport process has
permitted partial dissection of the system. We are, however, very far from
completely understanding the actual mechanisms involved in chemiosmotic
systems.
The binding protein systems represent another type
of transport through membranes. These systems utilize proteins located in the
periplasmic space that specifically bind sugars, amino acids, and ions.
Apparently, these periplasmic binding proteins transfer their substrates to
specific carrier molecules located in the cell membrane. The energy source for
these systems is ATP or a closely related metabolite.
Transporting large molecules through the cell wall
and membranes poses additional problems. Eukaryotic cells can move larger
molecules through the membrane by exocytosis and endocytosis processes in which
the membrane encompasses the molecule or molecules. In the case of endocytosis,
the molecule can enter the cell, but it is still separated from the cytoplasm
by the membrane. This membrane must be removed in order for the membrane-enclosed
packet of material to be released into the cytoplasm. By an analogous process,
exocytosis releases membrane-enclosed packets to the cell exterior.
Releasing phage from bacteria also poses difficult
problems. Some types of filamentous phage slip through the membrane like a
snake. They are encapsidated as they exit the membrane by phage proteins
located in the membrane. Other types of phage must digest the cell wall to make
holes large enough to exit. These phage lyse their hosts in the process of
being released.
An illuminating example of endocytosis is the
uptake of low density lipoprotein, a 200 ├ģ diameter protein complex that
carries about 1,500 molecules of cholesterol into cells. Pits coated with a
receptor of the low density lipoprotein form in the membrane. The shape of
these pits is guided by triskelions, an interesting structural protein
consisting of three molecules of clathrin. After receptors have been in a pit
for about
Figure
1.9 Endocytosis of receptor-coated
pits to form coated vesicles and therecycling of receptor that inserts at
random into the plasma membrane and then clusters in pits
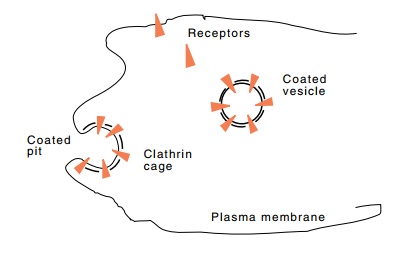
ten minutes, the pit pinches off and diffuses
through the cytoplasm (Fig. 1.9). Upon reaching the lysosome, the clatherin
cage of triskelions is disassembled, cholesterol is released, and the receptors
recycle.
Related Topics