Chapter: Modern Analytical Chemistry: Kinetic Methods of Analysis
Methods Based on Chemical Kinetics: Quantitative Applications
Quantitative Applications
Chemical kinetic methods
of analysis continue
to find use for the analysis of a vari- ety of analytes, most notably in clinical laboratories, where automated methods
aid in handling a large volume of samples.
In this section several general
quantitative applications are considered.
Enzyme-Catalyzed Reactions
Enzymes are highly specific catalysts for biochemi- cal reactions, with each enzyme
showing a selectivity for a single
reactant, or sub-
strate. For example,
acetylcholinesterase is an enzyme that catalyzes the decomposi-
tion
of the neurotransmitter acetylcholine to choline and acetic acid. Many enzyme–substrate reactions follow a simple
mechanism consisting of the initial
for- mation of an enzyme–substrate complex, ES, which subsequently
decomposes to form product, releasing the enzyme to react again.

If measurements are made early
in the reaction, the product’s concentration is neg- ligible, and the step described by the rate constant k–2 can be ignored.
Under these conditions the rate of the reaction
is

To be analytically useful equation 13.16
needs to be written in terms of the
concentrations of enzyme and substrate. This is accomplished by applying
the “steady-state” approximation, in
which we assume
that the concentration of ES is essentially constant. After an initial period
in which the enzyme–substrate complex first forms, the rate of formation of ES
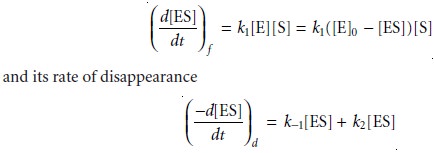
are equal. Combining these equations gives
k1([E]0 – [ES])[S] = k–1[ES] + k2[ES]
which is solved
for the concentration of the enzyme–substrate complex
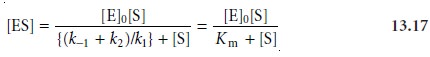
where Km is called the Michaelis
constant. Substituting equation
13.17 into equa- tion 13.16 leads to the final rate equation

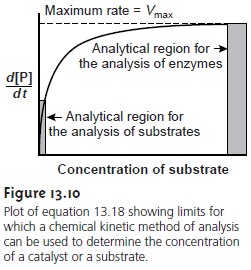
A plot of equation 13.18,
shown in Figure
13.10, is instructive for defining con- ditions under which the rate of an enzymatic
reaction can be used for the quantita- tive analysis of enzymes
and substrates. For high substrate
concentrations, where [S] >> Km,
equation 13.18 simplifies to
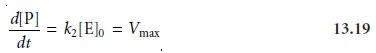
where Vmax is the maximum
rate for the catalyzed reaction. Under these conditions the rate of the reaction is pseudo-zero-order in substrate, and the maximum
rate can be used
to calculate the
enzyme’s concentration. Typically, this determination is made by a variable-time method. At lower substrate concentrations, where
[S] << Km,
equation 13.18 becomes
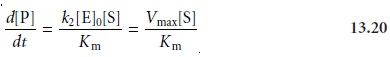
The reaction is now first-order in substrate, and the rate of the reaction can be used to
determine the substrate’s concentration by a fixed-time method.
Chemical kinetic methods have been applied to the quantitative
analysis of a number of enzymes and substrates.13 One example, is the determination of glucose
based on its oxidation by the enzyme
glucose oxidase.
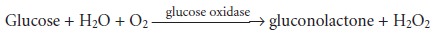
Conditions are controlled, such that equation
13.20 is valid. The reaction
is moni- tored by following the rate of change in the concentration of dissolved O2 using an
appropriate voltammetric technique.
Nonenzyme-Catalyzed Reactions
The variable-time
method has also been used to determine the concentration of nonenzymatic catalysts. Because a trace
amount of catalyst can substantially enhance
a reaction’s rate,
a kinetic determination of a cat- alyst’s concentration is capable
of providing an excellent detection limit. One of the
most commonly used
reactions is the
reduction of H2O2 by reducing agents,
such as thiosulfate, iodide,
and hydroquinone. These reactions are catalyzed by trace levels of
selected metal ions.
For example the reduction of H2O2 by I–
2I– + H2O2 + 2H3O+ < = = = = > 2H2O+ I2
is catalyzed by Mo(VI), W(VI), and Zr(IV).
A variable-time analysis
is conducted by adding
a small, fixed
amount of ascorbic
acid to each solution. As I2 is produced,
it rapidly oxidizes the ascorbic
acid and is, itself, reduced
back to I–. Once all the
ascorbic acid is consumed, the presence of excess I2 provides a visual
end point.
Noncatalytic Reactions
Chemical kinetic methods are not as common for the quantitative analysis of analytes in noncatalytic reactions. Because they lack
the en- hancement of reaction rate obtained when using a catalyst, noncatalytic methods generally are not used for the determination of analytes at low concentrations.4 Noncatalytic methods
for analyzing inorganic analytes are usually
based on a com-
plexation reaction. One example was outlined in Example 13.4,
in which the con-
centration of aluminum in serum was determined by the initial
rate of formation of its
complex with 2-hydroxy-1-naphthaldehyde p-methoxybenzoyl-hydrazone.10 The greatest
number of noncatalytic methods, however, are for the quantitative
analysis of organic analytes. For example, the insecticide methyl
parathion has been determined by measuring its
rate of hydrolysis in alkaline solutions.
Related Topics