Chapter: Modern Analytical Chemistry: Kinetic Methods of Analysis
Methods Based on Chemical Kinetics: Characterization Applications
Characterization Applications
Chemical kinetic methods
also find use
in determining rate
constants and elucidat- ing reaction mechanisms. These applications are illustrated by two examples
from the chemical kinetic
analysis of enzymes.
Determining Vmax and Km for Enzyme-Catalyzed Reactions
The value of Vmax and Km for an enzymatic reaction are of significant interest
in the study of cellular chemistry.15 From equation 13.19 we see that Vmax provides a means
for determin- ing the rate constant
k2. For enzymes that follow the mechanism shown in reaction 13.15, k2 is equivalent to the enzyme’s turnover number, kcat. The
turnover number is the
maximum number of substrate molecules converted to product
by a single ac- tive site on the enzyme, per unit time. Thus, the turnover number provides a direct
indication of the catalytic efficiency of an enzyme’s
active site. The Michaelis con- stant, Km, is significant because
it provides an estimate of the substrate’s intracellu- lar concentration.
Values of Vmax
and Km for reactions
obeying the mechanism shown in reaction 13.15 can be determined using equation 13.18
by measuring the rate of reaction as a
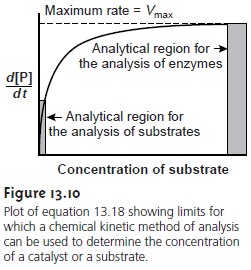
function of the substrate’s concentration. The curved nature of the relationship be- tween
rate and the
concentration of substrate (see Figure 13.10),
however, is incon- venient for this purpose.
Equation 13.18 can be rewritten in a linear
form by taking its reciprocal
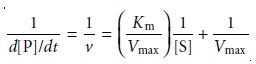
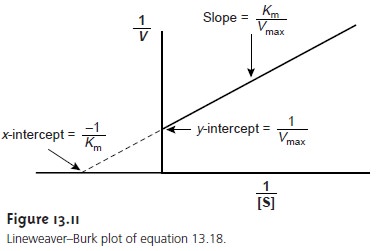
A plot of 1/v versus 1/[S],
which is called
a double reciprocal, or Lineweaver–Burk plot, is
a straight line
with a slope
of Km/Vmax, a y-intercept of 1/Vmax, and an x-intercept of –1/Km (Figure 13.11).
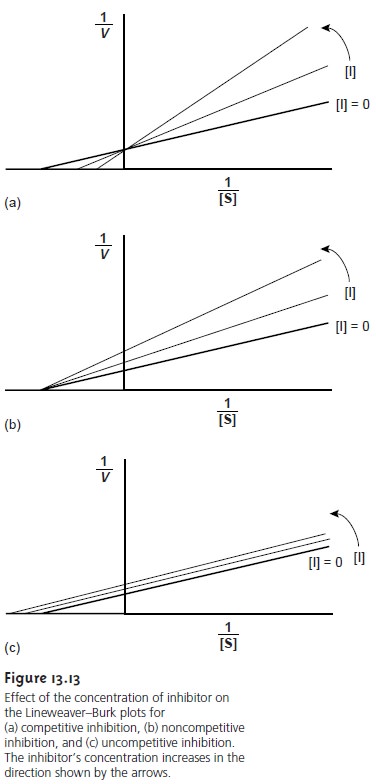
Elucidating Mechanisms for the Inhibition of Enzyme Catalysis
An inhibitor inter- acts with an enzyme in a manner that decreases the enzyme’s catalytic efficiency. Examples of inhibitors include some drugs and poisons. Irreversible inhibitors co- valently bind to the enzyme’s active site, producing a permanent loss in catalytic ef- ficiency even when the inhibitor’s concentration is decreased. Reversible inhibitors form noncovalent complexes with the enzyme, thereby causing a temporary decrease in catalytic efficiency. If the inhibitor is removed, the enzyme’s catalytic effi- ciency returns to its normal level.
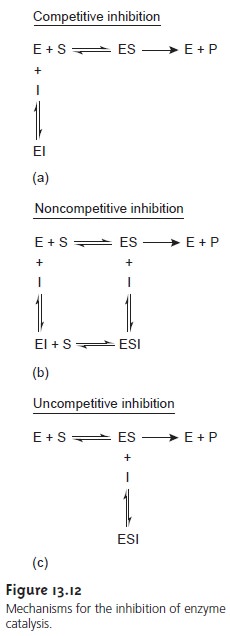
The reversible binding
of an inhibitor to an enzyme can occur through
several pathways, as shown in Figure 13.12. In competitive inhibition (Figure 13.12a), the substrate and the inhibitor compete for the same active
site on the enzyme. Because the substrate cannot bind
to an enzyme that is already bound
to an inhibitor, the
enzyme’s catalytic efficiency for the substrate is decreased. With
noncompetitive in- hibition (Figure
13.12b) the substrate
and inhibitor bind to separate
active sites on the enzyme, forming
an enzyme–substrate–inhibitor, or ESI complex.
The presence of the ESI complex,
however, decreases catalytic efficiency since only the enzyme–substrate complex
can react to form product.
Finally, in uncompetitive in- hibition (Figure 13.12c)
the inhibitor cannot
bind to the free enzyme,
but can bind to
the enzyme–substrate complex.
Again, the result
is the formation of an inactive
ESI complex.
The three reversible mechanisms for enzyme
inhibition are distinguished by ob- serving how changing the inhibitor’s concentration affects the relationship between the rate of reaction and the concentration of substrate. As shown in Figure 13.13, when kinetic data are displayed as a Lineweaver–Burk plot, it is possible to determinewhich mechanism is in effect.
Related Topics