Chapter: Modern Pharmacology with Clinical Applications: Principles of Toxicology
Manifestations of Toxicity
MANIFESTATIONS
OF TOXICITY
Organ Toxicity
The events that initiate cell
death are not completely understood. The common final stages of necrotic cell
death are disruption of normal metabolic processes and ensuing inability to
maintain intracellular electrolyte homeostasis. If the insult is severe or
prolonged enough, the cell will not regain normal function. At the same time,
other cells show apoptotic cell death, character-ized by cell shrinkage,
cleavage of DNA between nucle-osomes, and formation of apoptotic bodies. Some
chem-icals are metabolized to reactive products that bind to cellular
macromolecules. If such binding impairs the function of crucial macromolecules,
cell viability is lost. How severely organ function will be impaired depends on
the reserve capacity of that organ. The ultimate out-come will depend on the
affected organ’s regenerative capacity and response to damage.
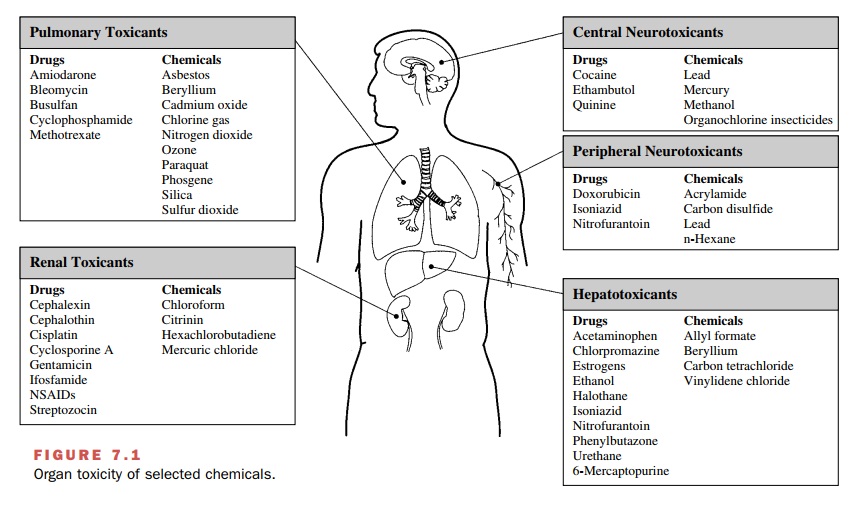
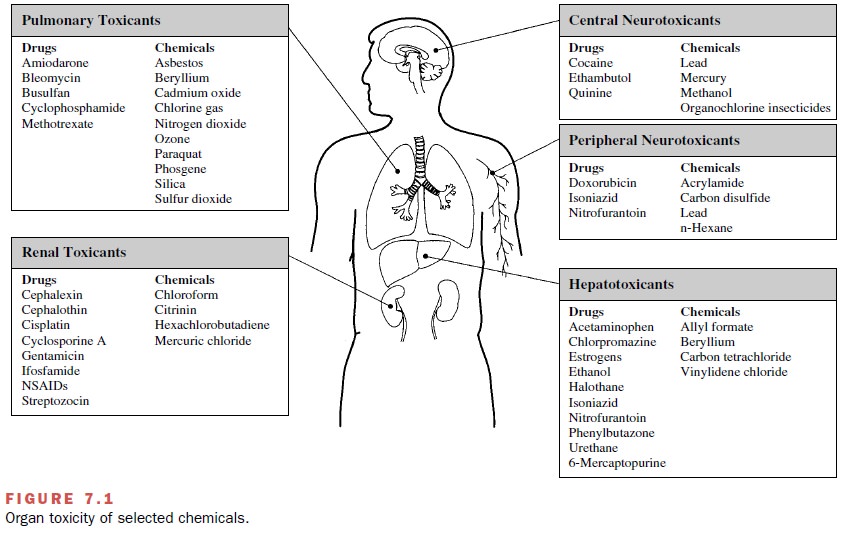
Pulmonary Toxicity
Inhaled gases, solid
particles, or liquid aerosols may de-posit throughout the respiratory system,
depending on their chemical and physical properties. The large surface area of
the respiratory passages and alveolar region and the large volume of air
delivered to that area (approxi-mately 6–7 L/minute in a young man) provide
great op-portunity for interaction between inhaled materials and lung tissue.
Examples of inhaled xenobiotics that cause lung damage and those that have entered the
body by ingestion, injection, or dermal absorption are presented in Figure 7.1.
Exposure of the lungs to
xenobiotics may result in a number of disease conditions including bronchitis,
em-physema, asthma, hypersensitivity pneumonitis, pneu-moconiosis, and cancer.
During repair, damaged lung alveolar epithelium may be replaced by fibrous
tissue that does not allow for gas exchange, which intensifies the damage
caused by the initial lesion.
Hepatotoxicity
The blood draining the stomach
and small intestine is delivered directly to the liver via the hepatic portal
vein, thus exposing the liver to relatively large concentrations of ingested
drugs or toxicants (e.g., Fig. 7.1). Hepatic ex-posure to agents that undergo
bioactivation to toxic species can be significant.
Hepatic necrosis can be
classified by the zone of the liver tissue affected. Xenobiotics, such as
acetamino-phen or chloroform, that undergo bioactivation to toxic intermediates
cause necrosis of the cells surrounding the central veins (centrilobular) because the compo-nents of the cytochrome P450
system are found in those cells in abundance. At higher doses or in the
presence of agents that increase the synthesis of cytochrome P450 (inducers),
the area of necrosis may incorporate the midzonal
area (midway between the portal triad and central vein). Cells around the portal triad are exposed to the
highest concentrations; necrosis occurs with direct-acting agents. A single
large dose of a hepato-toxin may cause liver necrosis yet resolve with little
or no tissue scarring. Continued exposure to the toxic agent, however, can
result in hepatic cirrhosis and per-manent scarring.
Allergic reactions to drugs
produce foci of necrosis that are scattered throughout the liver. Other agents cause
severe (chlorpromazine) or mild (estrogens) cholestatic liver damage, including
cholestasis and inflam-mation of the portal triad and hepatocellular necrosis.
Nephrotoxicity
The kidneys are susceptible
to toxicity from xenobiotics (Fig. 7.1) because they too have a high blood
flow. Cells of the tubular nephron face double-sided exposure, to agents in the
blood on the basolateral side and in the fil-tered urine on the luminal side.
Proximal tubule cells are generally the site of nephrotoxicity, since these
cells have an abundance of cytochrome P450 and can trans-port organic anions
and cations from the blood into the cells, thereby concentrating these
chemicals manyfold.
Chemically induced kidney damage is typically seen as acute tubular necrosis (ATN). The cells in the proxi-mal tubule are affected. Reabsorption of water, electrolytes, glucose, and amino acids is impaired.
Feedback mechanisms decrease glomerular filtration and thus
prevent delivery of large volumes of water to nephron segments. Urine output
may be increased, decreased, or unchanged. Markers of glomerular filtration, blood urea nitrogen (BUN) and creatinine, are increased only if
fil-tration falls by 80%. The urine may contain glucose and protein, including
proteinaceous casts formed in the nephron of tubular debris.
Neurotoxicity
Although the CNS is protected
from a number of xeno-biotics by the blood-brain barrier, the barrier is not
ef-fective against lipophilic compounds, such as solvents or insecticides (Fig.
7.1). Similarly, the peripheral nervous system is protected by a blood-neural
barrier. The bar-riers are less well developed in the immature nervous system,
rendering the fetus and neonate even more sus-ceptible to neurotoxicants.
Neural tissue susceptibility is due in large part to its high metabolic rate,
high lipid content, and for the CNS, high rate of blood flow.
Since damaged neural tissue
cannot easily replicate, glial and other nonconducting cells may proliferate
and occupy the space of the dead neurons, and the damage may be expressed as
deficits of sensory and motor func-tions and behavior. Alternatively, other
neurons may take on the functions of the damaged neurons such that there is
little or no perceptible damage.
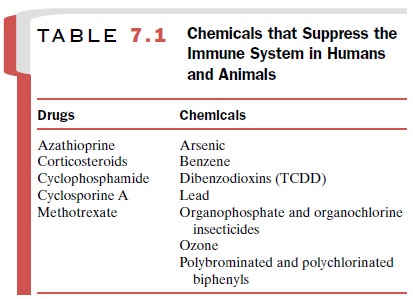
Immunotoxicity
A number of drugs and environmentally
and occupa-tionally important chemicals can impair the activity of one or more
components of the immune system. Immunodeficiency may result in increased
susceptibility to infection, decreased surveillance against precancer-ous or
cancerous cells, or tissue-damaging reactions (Table 7.1). Allergic and
autoimmune reactions are ex-amples of this form of toxicity.
Clinical expressions of
cutaneous allergic reactions include eczematous, indurate–inflammatory, and
ur-ticarial eruptions. Irritant responses causing direct dam-age to the skin
may be confused with allergic responses involving immune mechanisms. An
important differ-ence is that allergic reactions require an initial exposure to
sensitize the individual; dermatitis is then elicited by minimal subsequent
exposure to the agent.
Toxic Effects on Genetic Material and Cell Replication
Mutagenesis, teratogenesis, and carcinogenesis are dif-ferent manifestations of damage to genetic material (genotoxicity). Chemically induced genotoxicity occurs in several steps, and at each step there is opportunity for repair.
Generally, xenobiotics are not themselves mutagenic, but rather they must
be bioactivated to metabo-lites that are sufficiently reactive to bind to DNA
and disrupt its coding. The reactive intermediates must be formed close enough
to the DNA to interact with it be-fore interacting with other less important
macromole-cules or before being further metabolized to inactive forms.
Nongenotoxic carcinogens act by altering cell replication control.
Reproductive Toxicity
Most drugs and chemicals pose
a threat to the develop-ing fetus. An estimated 4 to 5% of developmental
de-fects in humans result from prenatal exposure to drugs or environmental
chemicals. This is particularly impor-tant, since women with irregular
menstrual cycles may be exposed to teratogens and enter the sensitive period of
organogenesis before pregnancy is
suspected.
Gestation is generally
considered to consist of three periods of development, each with differing sensitivities
to chemicals. During the preimplantation
or prediffer-entiation phase, expression of toxicity is an all-or-none
phenomenon; damage to the embryo results in either death or no effect. Organogenesis occurs during the
em-bryonic period (the first 3 months of pregnancy), and therefore,
susceptibility to teratogenesis is high; the em-bryo is particularly vulnerable
to teratogens on days 25 through 40. The fetal
period consists of the last 6 months of gestation and is a time of reduced
susceptibility to teratogenic alterations. Certain organs, such as the
gen-itals and the nervous system, however, are still under-going
differentiation during this period. Functional im-pairment in tissues without
marked structural damage and growth retardation is the most common effect of
chemical exposure during the fetal period.
Chemicals such as
1,2-dibromo-3-chloropropane can disrupt spermatogenesis, leading to impaired
repro-ductive function, including sterility. Men and women undergoing cancer
chemotherapy with alkylating drugs are at increased risk for sterility.
Related Topics