Chapter: Basic Radiology : Scope of Diagnostic Imaging
Magnetic Resonance Imaging
MAGNETIC RESONANCE IMAGING
In 1952, Felix Bloch and Edward
Purcell were awarded the Nobel Prize for their independent discovery of the
magnetic resonance phenomenon in 1946. Between 1950 and 1970, nuclear magnetic
resonance (NMR) was developed and used for chemical and physical molecular
analysis. In 1971, Raymond Damadian demonstrated that NMR had utility in cancer
di-agnosis, based on prolonged relaxation times in pathologic tissue. The first
2D proton NMR image of a water sample was generated in 1972 by Paul Lauterbur
using a back-projection technique, similar to that used in CT. In 1975, Richard
Ernst used phase and frequency encoding, as well as Fourier trans-form
analysis, to form the basis of current magnetic resonance imaging (MRI)
techniques. All of these experiments used de-fined, nonuniform magnetic fields
or linear variations in field strength along all coordinate axes. The
application of these nonuniform fields (magnetic field gradients) permitted
dis-crimination of various signals from different spatial loca-tions. In MR
imaging, a pulsed radiofrequency (rf) beam is used in the presence of a strong
main magnetic field to gen-erate high-quality images of the body. These images
can be acquired in virtually any plane, although sagittal, coronal, and axial
images are commonly obtained.
Although a detailed explanation
is beyond the scope of this topic, substances (eg, fluid) that have a long Tl
will appear dark on Tl-weighted images, whereas those with short Tl (fat) will
display high signal intensity. On T2-weighted images, a long-T2 substance
(fluid) will appear bright. Advantages of MR imaging include superb contrast
resolution, high spatial resolution, and lack of ionizing radiation.
The most commonly used clinically
approved contrast agents for MR imaging are gadolinium-based compounds that
produce T1 shortening. Tissue relaxation results from interactions between the
unpaired electron of gadolinium and tissue hydrogen protons, which significantly
decrease the T1 of the blood relative to the surrounding tissues. Adverse
reactions to this agent are far less frequent than those seen with iodinated
compounds, with common reactions includ-ing nausea, vomiting, headache,
paresthesias, or dizziness.
Hydrogen nuclei are favored for
MR imaging. On place-ment of a patient in an MR scanner, the randomly oriented
hydrogen nuclei align with the static magnetic field. In order to detect a
signal, a perturbing rf pulse is transiently applied to the patient, resulting
in a net change in alignment of these nu-clei. When the rf pulse is turned off,
the spins return to their equilibrium state by dissipating energy to the
surrounding molecules. The rate of energy loss is mediated by the intrinsic
relaxation properties of the tissue, designated as the longitudi-nal (T1) and
transverse (T2) relaxation times. T1 represents the restoration of the
longitudinal magnetization along the axis of the main magnetic field, whereas
T2 represents the decay time of the magnetization in the transverse plane.
Technical advances in gradient
hardware have resulted in faster and stronger gradients that permit subsecond
image scan times. Newer pulse sequences have been developed that currently
augment conventional MR pulse sequences (spin echo and gradient echo),
increasing the sensitivity of clini-cal studies to disease detection. These
rapid imaging tech-niques offer major advantages over conventional MR imaging,
including decreased image acquisition times, minimized pa-tient discomfort, and
increased ability to image physiologic processes in the body. In addition,
single-breath-hold scanning can be performed, reducing respiratory artifact.
Fast spin echo, fast gradient
echo, diffusion imaging, per-fusion imaging, and echo planar imaging (EPI) are
examples of fast imaging techniques that can be performed on clinical scanners.
Diffusion-weighted imaging is exquisitely sensitive to the microscopic
molecular motion of water, demonstrating areas of limited (restricted)
intracellular diffusion following an acute ischemic event. This sequence is
utilized routinely in clinical neuroimaging protocols but is somewhat
nonspecific for pathology, as diffusion changes that are characteristic of
acute ischemia can be observed with infection and some tumors.
Perfusion-weighted MRI, a less frequently used tech-nique, provides information
about the blood supply to a par-ticular area of the brain following rapid bolus
injection of gadolinium-based contrast agent. Echo planar imaging allows the
collection of all data required for image reconstruction in a fraction of a
second, after a single rf pulse. This technology has resulted in significant
clinical and scientific advances, such as in stroke evaluation and functional
brain imaging, re-spectively. Functional MRI studies of the human brain using
EPI techniques have allowed physiological investigations of the functional
organization of the brain.
MR angiography includes
contrast-enhanced MR an-giography and non-contrast-enhanced MR angiography.
Three-dimensional contrast-enhanced magnetic resonance angiography (MRA) is
used for noninvasive assessment of many vascular abnormalities, including aneurysms,
dissec-tion, vessel anomalies, and coarctation. It has evolved from the use of
fast scanning techniques on high-gradient-strength units, in combination with
contrast. Using this technique, volumetric acquisitions can be performed in a
single breath hold. Improvements in contrast resolution are achieved,
regardless of the plane of acquisition. This has allowed reductions in the
number of image sections needed to display a large vascular territory, as well
as over-all imaging acquisition times. Multiphase dynamic imaging is usually
performed after intravenous gadolinium admin-istration, with the arteries best
seen during the early phase and veins during the later phases. Noncontrast MRA
methods, such as 3D time-of-flight (TOF) MR, is used to evaluate intracranial
arterial (Figure 1-8) and carotid arteries. In addition, 2D TOF MR imaging is
used to evaluate periph-eral vascular diseases.
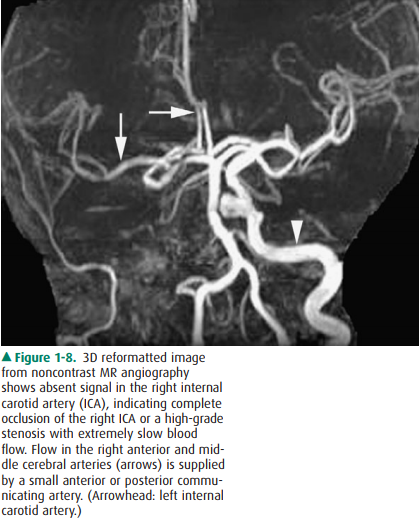
Figure 1-8. 3D reformatted image from noncontrast MR angiography shows absent signal in the right internal carotid artery (ICA), indicating complete occlusion of the right ICA or a high-grade stenosis with extremely slow blood flow. Flow in the right anterior and mid-dle cerebral arteries (arrows) is supplied by a small anterior or posterior commu-nicating artery. (Arrowhead: left internal carotid artery.)
Clinical Applications
MRI has traditionally been used
for neurologic indications, including brain tumors (Figure 1-9), acute
ischemia, infec-tion, and congenital abnormalities. MRI has been used for a
number of nonneurologic indications, namely, spine, muscu-loskeletal (MSK),
cardiac, hepatic, biliary, pancreatic, adre-nal, renal, breast, and female
pelvic imaging. Spine MR studies are useful for evaluating degenerative
changes, disk herniation, infection, metastatic disease, and congenital
ab-normalities. Common MSK applications involve imaging of large joints, such
as knee, shoulder, and hip. The primary common indication for MRI of the knee
is the assessment of menisci and ligaments following internal derangement.
Rotator cuff tear is a typical shoulder indication. Cardiac studies are
performed to identify complex malformations, cardiac func-tion, myocardiac
viability, valvular disease, myocardial perfu-sion, and congenital heart
disease. In the abdomen, hepatic MRI studies are often used to diagnose
atypical presentations of liver lesions, metastatic disease, and hepatocellular
carci-noma. Adrenal studies are performed primarily to distin-guish adrenal adenomas
from metastatic disease. Atypical renal masses, found incidentally on US or CT,
can often be better characterized on MRI. In addition, renal MRI is used to
establish the presence and extent of tumor thrombus in cases of renal-cell
carcinoma for tumor staging purposes. Breast MRI is utilized to stage cancer,
to screen patients at high risk, to look for unknown primary cancer in patients
with positive axillary nodes, for delineation of residual cancer after
chemotherapy, and sometimes for patients with equivo-cal mammographic and/or US
findings. Finally, oncologic applications in the female pelvis include the
diagnosis and characterization of cervical and endometrial carcinomas, as well
as adnexal lesions. MR enterography is used in the eval-uation of small-bowel
disease (Figure 1-10).
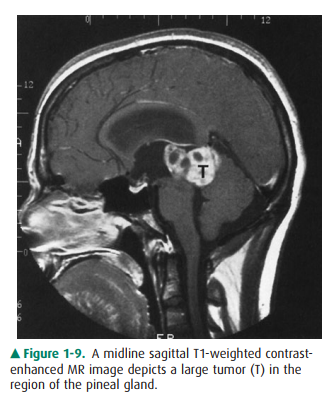
Figure 1-9. enhanced MR region of the A midline sagittal T1-weighted
contrast-image depicts a large tumor (T) in the pineal gland.
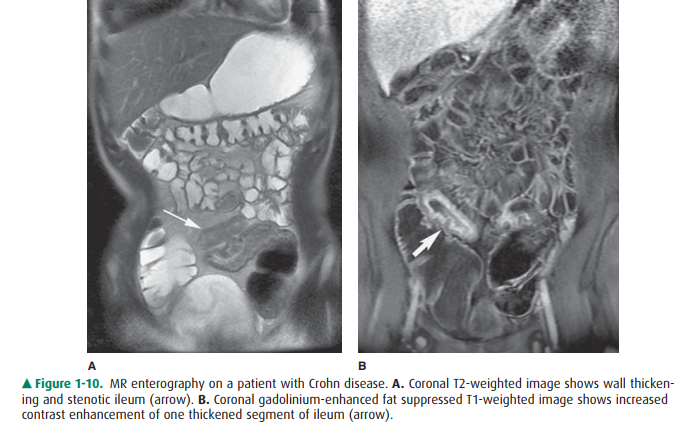
Figure 1-10. MR enterography on a patient with Crohn disease. A. Coronal T2-weighted image shows wall thicken-ing and stenotic ileum (arrow). B. Coronal gadolinium-enhanced fat suppressed T1-weighted image shows increased contrast enhancement of one thickened segment of ileum (arrow).
Magnetic Resonance Cholangiopancreatography
Magnetic resonance
cholangiopancreatography (MRCP) is used to evaluate choledocholithiasis,
retained gallstones, pancreatobiliary neoplasms, strictures, primary sclerosing
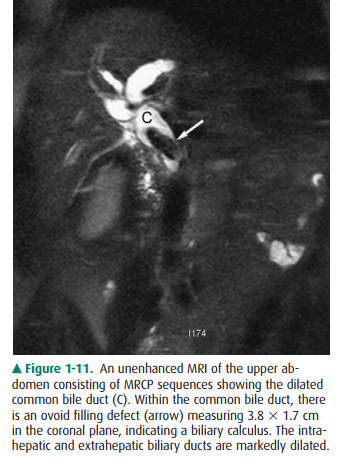
cholangitis, and chronic pancreatitis (Figure 1-11). This non-contrast
technique relies on the relatively stationary nature of bile (compared with
blood) to depict the predominantly fluid-filled pancreatic ducts and biliary tree.
Rapid heavily T2-weighted breath-hold sequences are utilized, resulting in
visualization of high signal-intensity ductal structures. In pa-tients who have
failed endoscopic retrograde cholangiopan-creatography (ERCP), or who are
unable to tolerate this procedure, MRCP has become a suitable alternative. MRCP
is particularly useful in postoperative patients, patients with biliary system
anomalies, and as a screening tool in patients with an otherwise low
probability of a biliary abnormality. ERCP is generally reserved for
therapeutic purposes, such as stent placement, stone extraction, or stricture
dilatation.
Nephrogenic Systemic Fibrosis
Since 2006, it has been reported
that the administration of gadolinium-based contrast agents for MR imaging is
associ- ated with the development of nephrogenic systemic fibrosis (NSF) in
some patients with renal insufficiency. Although NSF was first reported in
1997, the exact cause of develop-ment of NSF remains unknown. The dissociation
of gadolin-ium ion from the chelating ligand recently has been proposed as an
etiologic factor in the development of NSF. The inci-dent of NSF ranges from
0.003% to 0.039% depending on the report cited. The incidence of NSF may
increase to 1% to 7% in patients with severe chronic kidney disease following
ex-posure to gadolinium-based contrast media. All patients in published case
reports developed NSF within 6 months fol-lowing administration of
gadolinium-based contrast agent. The majority of patients with renal
insufficiency in these published reports, however, did not develop NSF
following administration of gadolinium chelates. The development of NSF
following the administration of a gadolinium chelate contrast has been reported
to be particularly associated with patients who have acute or chronic renal
disease with a glomerular filtration rate (GFR) lower than 30 mL/min/ 1.73 m2,
and in those with acute renal insufficiency. The esti-mated GFR was calculated
by using the patient’s age, weight, and race and serum creatinine level. Some
risk factors, such as concurrent proinflammatory conditions, metabolic
con-ditions including acidosis and high calcium-phosphate products, or
concurrent tissue injury, surgery, and is-chemia, are associated with the
development of NSF in pa-tients who underwent gadolinium-based contrast MR
imaging.
In 2007, the US Food and Drug
Administration (FDA) re-quested that a warning be added to all five
FDA-approved gadolinium-based contrast agents regarding the potential risk of
NSF in patients with renal failure. These five FDA-ap-proved products include
gadodiamide (Omniscan, GE Healthcare, Oslo, Norway), gadopentetate dimeglumine
(Magnevist, Bayer Healthcare, Wayne, NJ), gadobenate dimeglumine (MultiHance,
Bracco Diagnostics, Princeton, NJ), gadoteridol (ProHance, Bracco Diagnostics,
Princeton, NJ), and gadoversetamide (OptiMARK, Tyco-Mallinckrodt, St Louis,
MO). One recent recommendation aimed at de-creasing the risk of NSF has been to
use 0.1 mmol/kg of gadolinium contrast for patients with GFR lower than 30
mL/min. If a patient is in a dialysis program, some experts believe that it may
be prudent to dialyze after administration of gadolinium-based contrast agent.
Alternative imaging ex-aminations, such as arterial spin-labeling perfusion
MRI, may replace administration of gadolinium for some.
MR imaging is contraindicated for
patients with metal implants or foreign bodies, such as intracranial aneurysm
clips, intraorbital metallic foci, cardiac pacemakers, or spe-cific types of
cardiac valves. In these instances, these objects may be dislodged or damaged
by the magnetic field. MR im-aging may also be contraindicated for
claustrophobic or un-cooperative patients who may not respond to conscious
sedation protocols.
Related Topics