Chapter: Surgical Pathology Dissection : The Hematopoietic and Lymphatic System
Lymph Nodes : Surgical Pathology Dissection
Lymph Nodes
High-quality
sections for routine light micros-copy are necessary, but not always
sufficient, for the interpretation of lymph node biopsies. Immu-nophenotypic
and genetic studies are often re-quired for the diagnosis and classification of
a hematopoietic neoplasm. Adequate fixation and timely and appropriate
technical handling of lymph nodes are, therefore, even more important than with
other specimens.
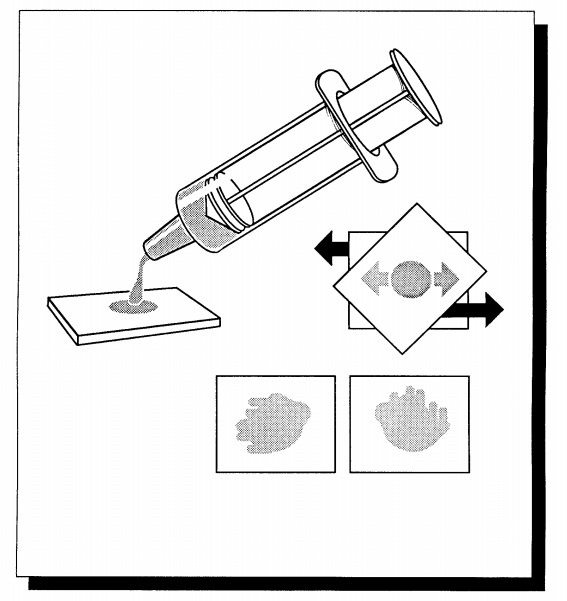
When
lymph nodes are placed in an empty specimen container or in dry gauze, the
edges of the specimen dry out, producing a prominent desiccation artifact at
the edge of the node. Severe edge artifacts can be introduced into a lymph node
even before the specimen reaches the surgi-cal pathology laboratory. Surgeons
should there-fore be instructed to place resected lymph nodes immediately into
a balanced physiologic solution such as Roswell Park Memorial Institute medium
(RPMI) 640 or isotonic saline, and to transport lymph nodes immediately to the
surgical pathol-ogy laboratory. Remember that lymph nodes can also dry out on
the cutting table, so proceed quickly and efficiently after removing the
speci-men from the transport media.
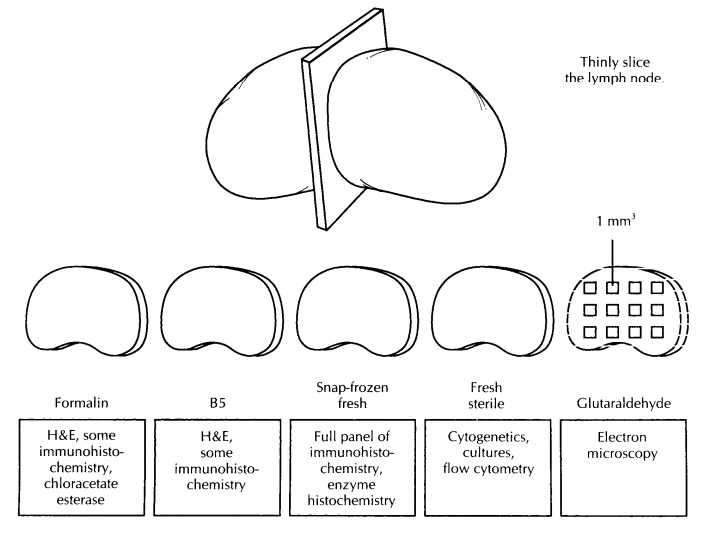
Once the
specimen is received, document its size, weight, and shape, and then slice it
into uniformly thin 2- to 3-mm sections. Examine the cut surfaces of the node,
and ask the following questions: Is the nodal architecture preserved? If the
architecture is ablated, is the node grossly nodular, or is the process
diffuse? Are any focal lesions present? Is the capsule intact? What is the
appearance of the perinodal tissues?
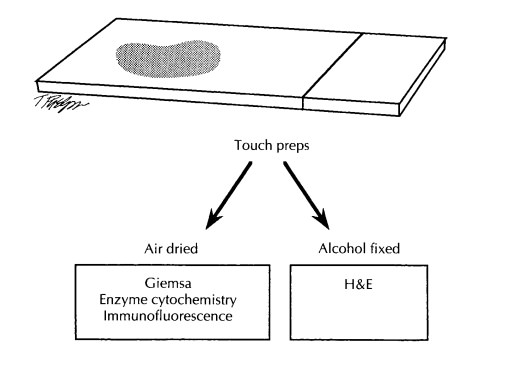
Next,
prepare touch imprints by placing the surface of a glass slide against the cut
surface of the lymph node. At least five air-dried slides should be prepared,
especially in cases of sus-pected Burkitt’s lymphoma, lymphoblastic lym-phoma,
and myelogenous leukemia. These can be used later for Giemsa stains, oil red O
stains, acid phosphatase stains, chloracetate esterase stains, and
immunofluorescence for nuclear terminal transferase. Two additional imprints immediately
fixed in 95% alcohol should be prepared for possible hematoxylin and eosin
(H&E) staining.
Next,
tissue should be submitted for light mi-croscopy and, if sufficient tissue is
available, for immunohistochemical and genetic studies. Sec-tions for light
microscopy should include not only the substance of the node, but also the
capsule and perinodal soft tissues. Submit at least one section for fixation in
neutral buffered formalin and at least one section in B-5 or an equivalent
fixative. The B-5 fixative contains mercuric chlo-ride as well as formaldehyde,
and it provides crisp nuclear detail. If a section is submitted in a
mercury-based fixative, remember to notify your tissue processing laboratory
personnel because these sections require special processing.
When
submitting fresh tissue for special stud-ies, collect the sample from solid
‘‘fleshy’’ areas of the tumor. Avoid areas that appear necrotic or sclerotic as
these areas may not contain a sufficient quantity of viable tumor cells. The
best techniques for submitting fresh tissue for im-munophenotyping will depend
on your individ-ual laboratory, but in general a representative section of the
node should be snap-frozen in opti-mal controlled temperature embedding medium
for frozen tissue specimens (OCT) for immuno-histochemical studies, and a
separate 0.5- to 0.7-cm cube should be submitted fresh for flow cytometry.
Again, the rapid handling of tissue for these studies is crucial, because
delays canresult in diffusion artifacts during immuno-staining. If tissue will
be sent off-site for these analyses, it should not be frozen, but instead it
should be kept cool on ice and rapidly trans-ported.
If
adequate tissue is available, and it usually is, fresh tissue should also be
sent for genetic studies such as gene rearrangements and kar-yotyping.
Submitting this tissue is important because antigen receptor gene rearrangement
analysis may be required in those rare cases for which morphology and
immunohistochemistry alone cannot establish the diagnosis. Obtain in-structions
on how to submit these specimens properly from your genetics laboratory.
Finally,
if an infection is suspected or granu-lomas are encountered on a preliminary
frozen section evaluation, fresh sterile tissue should be submitted for microbiologic
studies.
Important Issues to Address in Your Surgical Pathology Report on Lymph Nodes
• What
procedure was performed, and what structures/organs are present?
• Anatomically,
from where were the lymph nodes removed?
• What are
the type and grade of the neoplasm?
• What are
the number and size of the lymph nodes involved by tumor?
• What
special studies were performed, and what were the results of these studies?
Extranodal Specimens
The lymphatic system is not limited to lymph nodes but encompasses diverse tissues and organs including the spleen, thymus, bone marrow, Waldeyer’s ring, vermiform appendix, and mucosa-associated lymphoid tissue of the intestines and lung. Lymphomas can arise anywhere in this rather extensive lymphatic system. Moreover, they can arise in extranodal sites that are not part of the lymphatic system (e.g., thyroid, stomach).
If the
nature of a tumor is unknown at the time of specimen processing, a touch prep
or frozen section of the tumor is a fast, simple way to determine if you are
dealing with lymphoid proliferation. This is important information to have as
you begin the dissection because extra-nodal lymphoid proliferations, like
their nodal counterparts, need to be submitted for special studies as
appropriate. Once tissue has been ob-tained for special studies, the specimens
canthen be routinely processed in an organ-specific manner. There is no need to
modify your ap-proach in any significant way. Similar to dealing with some
epithelial neoplasm, remember to document the dimensions of the tumor,
deter-mine the degree of involvement of adjacent struc-tures, assess the status
of the surgical margins, and evaluate the regional lymph nodes. The un-involved
tissues should also be sampled, and any additional pathologic processes (e.g., Helico-bacter pylori infections in
stomach resections,thyroiditis in thyroid resections) should be in-cluded in
the final pathology report.
Related Topics