Chapter: Fiber optics and Laser instruments : Laser Fundamentals
Level Lasers
Level Lasers
Every
atom or molecule in nature has a specific structure for its energy levels. The
lowest energy level is called the ground state, which is the naturally
preferred energy state. As long as no energy is added to the atom, the electron
will remain in the ground state. When the atom receives energy (electrical
energy, optical energy, or any form of energy), this energy is transferred to
the electron, and raises it to a higher energy level (in our model further away
from the nucleus).The atom is then considered to be in an excited state. The
electron can stay only at the specific energy states (levels) which are unique
for each specific atom. The electron cannot be in between these "allowed
energy states", but it can "jump" from one energy level to
another, while receiving or emitting specific amounts of energy.
These
specific amounts of energy are equal to the difference between energy levels
within the atom. Each amount of energy is called a "Quantum" of
energy (The name "Quantum Theory" comes from these discrete amounts
of energy). Energy transfer to and from the atom Energy transfer to and from the
atom can be performed in two different ways:
1. Two-Level Laser
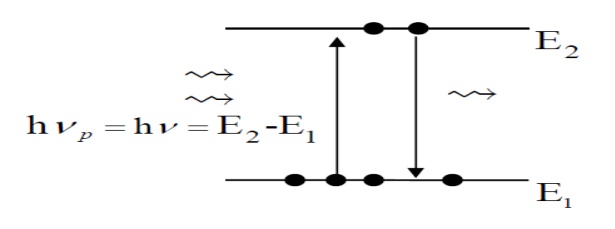
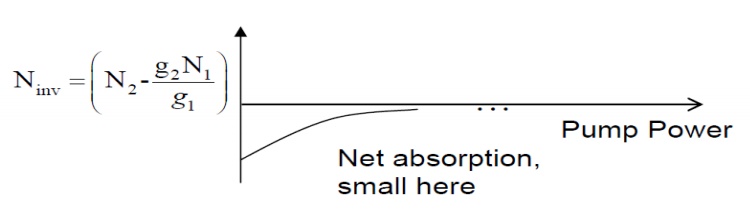
Suppose
we try to increase N2 with strong light at hν to create a population inversion
2. Three Level Laser
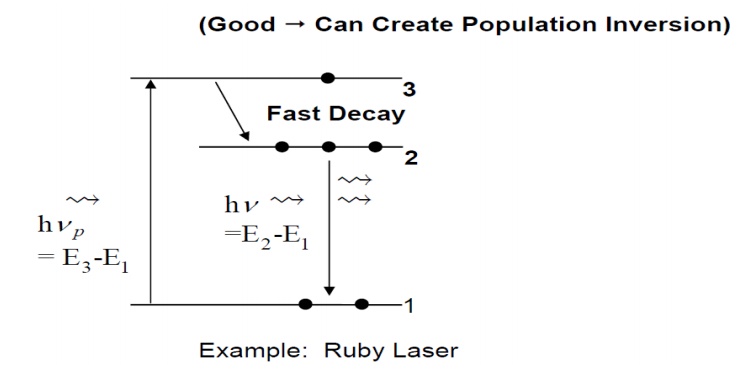
In a
three level system, the terminal level for the fluorescence process is the
ground level(ie) the level with the lowest energy. Here, The population
inversion is produced by raising electrons to the high energy level by the
process of pumping with an auxiliary light source. It is observed to excite
electrons from level 1 to level 3. Then , a very fast radiation less transition
accomplished by thermal vibrations of the atoms will drop the electrons to
level 2. The difference in energy between levels 3 and 2 appears as heat.
Stimulated emission occurs between levels 2 and 1 at frequency,
It
substantial power at frequency f3is supplied, the transition rate
from level 1 to 3 will be large.
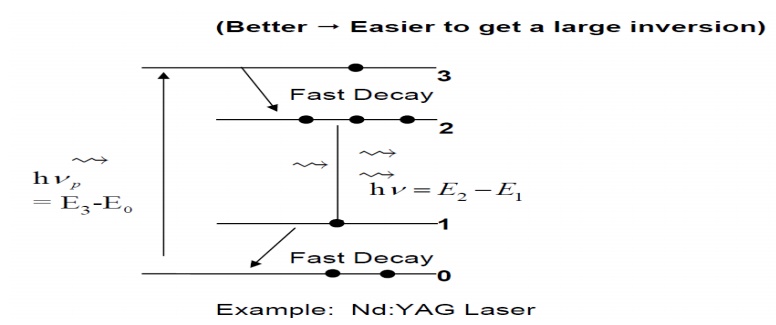
3. Quasi Three Level Laser
1.Collisions with other atoms, and the transfer of
kinetic energy as a result of the collision. This kinetic energy is transferred
into internal energy of the atom.
2.
Absorption and emission of electromagnetic radiation. Since we are now
interested in the lasing process, we shall concentrate on the second mechanism
of energy transfer to and from the atom (The first excitation mechanism is used
in certain lasers, like Helium-Neon, as a way to put energy into the laser.
The
interactions between electromagnetic radiation and matter cause changes in the
energy states of the electrons in matter.
•Electrons
can be transferred from one energy level to another, while absorbing or
emitting a certain amount of energy. This amount of energy is equal to the
energy difference between these
two
energy levels (E2-E1).
•When
this energy is absorbed or emitted in a form of electromagnetic radiation, the
energy difference between these two energy levels (E2-E1) determines uniquely
the frequency (ν) of theelectromagnetic radiation: ( ∆E) = E2-E1= hν= h(bar)ω
Eg:The laser is a system that is
similar to an electronic oscillator. An Oscillator is a system that produces oscillation s without an
external driving mechanism.
Related Topics