Chapter: Microbiology and Immunology: Bacteriology: Mycobacterium tuberculosis
Laboratory Diagnosis of Mycobacterium tuberculosis Infections
Laboratory Diagnosis
The clinical diagnosis of tuberculosis is supported by labora-tory diagnosis and other tests including radiographic evidence of pulmonary disease. Definitive diagnosis of tuberculosis is made by detection of M. tuberculosis from clinical specimens by microscopy or culture.
◗ Specimen
Collection of the specimen depends on the nature of the infection whether pulmonary or extrapulmonary. Sputum, lung tissue, gastric lavage, and bronchoalveolar lavage are the specimens collected for diagnosis of pulmonary tuberculosis. Cerebrospinal fluid (CSF), pleural fluid, peritoneal fluid, urine, lymph node tissue, bone marrow and blood are the other specimens frequently used in the diagnosis of extrapulmonary tuberculosis. In general, few tubercle bacilli are present in extra-pulmonary specimens than in sputum.
1. Sputum is the specimen of choice for pulmonary tuberculosis. Sputum, not saliva, is collected in the morn-ing into a clean wide-mouthed container, such as sputum cup. Collection of morning sputum specimen is ideal. If sputum is scanty, 24-hour specimen may be collected.
Sputum specimen collected on three consecutive days increases the chance of detection of tubercle bacilli.
2. Gastric aspirate may be used in place of sputum, especially in young children who cannot produce the sputum. In older children, bronchial secretions may be collected by stimulation of the cough by using an aerosol solution of propylene glycol in 10% sodium chloride. Bronchoalveolar lavage may also be used for the diagnosis of pulmonary tuberculosis.
3. Urine is the specimen of choice for diagnosis of genito-urinary tuberculosis. This is collected either as three con-secutive early morning samples or as a single sample of completely voided urine in 24 hours.
The urine specimens are collected in large, clean, ster-ile containers of 500 mL or more capacity and sent to the laboratory for processing. The urine specimens are centri-fuged at 300 rpm for 30 minutes, and the sediments are used for culture on selective media for M. tuberculosis.
4. CSF is collected for diagnosis of tubercular meningitis. The CSF is centrifuged and the sediment after centrif-ugation is stained for smears and is inoculated on the media for culture. CSF on standing for a long time devel-ops a spider web, the examination of which shows more bacilli.
5. Pleural fluid, peritoneal fluid, and other exudates are col-lected in containers with citrate to prevent coagulation. Bone marrow and liver tissue are usually collected from patients with miliary tuberculosis, and blood is collected from patients with HIV for isolation of bacteria by culture.
It is essential to collect all these specimens before starting antitubercular therapy. The specimens after collection should be transported as immediately as possible for staining and cul-ture. In case of delay, the specimens are refrigerated at 4°C, but not more than overnight. Further delay in processing decreases the possibility of isolation of tubercle bacilli by culture.
◗ Microscopy
Sputum microscopy is the most dependable and conventional method for demonstration of AFB by ZN staining method. The sputum smears are made by using new slides every time. The slides should not be reused, because AFB may adhere to the sur-face of the slide and may not be removed from the slide during the process of cleaning.
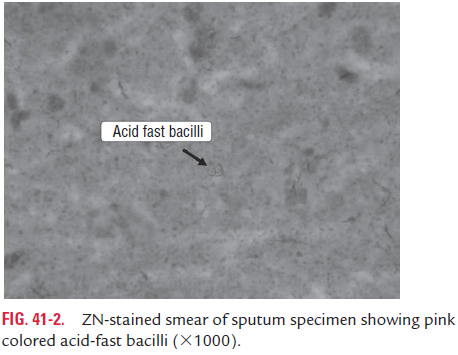
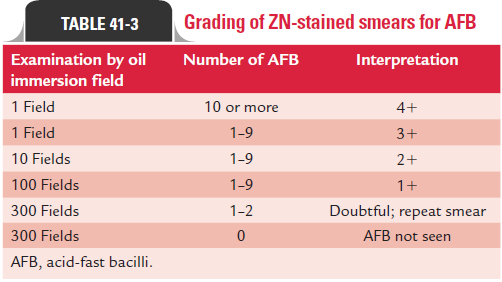
The smears are made from thick purulent part of the sputum and then are air dried, fixed by heating, and finally stained by ZN technique. The stained smear is examined under oil immer-sion lens (1003). Acid-fast bacteria in a stained smear appear bright red against a blue background. At least 50,000 to 1 lakh bacilli should be present per milliliter of sputum in order to be examined, taking about 10 minutes before giving negative report. The presence of at least two or more typical bacilli in the smear is reported as positive for AFB (Fig. 41-2, Color Photo 41).
The smears are graded depending on the number of tuber-cle bacilli present in a stained smear on examination by oil immersion lens.
Microscopic demonstration of AFB in stained smears is the presumptive diagnosis of tuberculosis. This is because ZN staining of AFB smears cannot be used to differentiate M. tuberculosis from other acid-fast organisms, such as sapro-phytic mycobacteria orNocardia species.
Saprophytic mycobacteria may present appearance simi-lar to that of M. tuberculosis. But nevertheless saprophytic mycobacteria stain uniformly without any barred or beaded appearance and are usually only acid fast but not alcohol fast. Moreover, these saprophytic mycobacteria are usually not present in the sputum and other respiratory secretions but are found in urogenital specimens, gastric aspirates, and fecal specimens.
Auramine–rhodamine stains are the fluorescent stains that are used as variation of the traditional ZN stain for demonstra-tion of AFB. In this method, the smears are stained with aura-mine–rhodamine or auramine–phenol fluorescent dyes and are examined by fluorescent microscope under ultraviolet illumi-nation. These AFB appear as bright fluorescing rods against a dark background. The bacilli, because of their contrast, are visualized even under a high power objective in contrast to oil immersion objective in ZN stain, thus enabling rapid screen-ing of the larger areas of the smear. These fluorescent-stained slides can be screened faster, because the AFB stand out against the nonfluorescent background. This is the major advantage of this staining method, hence is adopted in the laboratories where many smears are to be examined for AFB. However, it is always essential to confirm fluorochrome-positive smears by ZN staining.
The sputum microscopy may be negative in the early stage of the disease or in children in whom bacilli in respiratory secre-tions are few.
◗ Concentration of specimens
Concentration methods have been described for concentra-tion of mycobacteria in sputum and other specimens. By these methods, specimens are decontaminated and concentrated into a small volume without inactivation of the bacteria. The concentrated sediments are used for microscopy as well as for culture and animal inoculation. Several methods are now in use and include the following:
Petroff ’s method: It is a simple and widely used method. Inthis method, equal volume of sputum and 4% sodium hydrox-ide solution are mixed and incubated at 37°C with frequent shaking till it becomes clear, on an average for 20 minutes. It is then centrifuged at 3000 rpm for 30 minutes. The super-natant is discarded, and the sediment is neutralized by adding 8% hydrochloric acid. Phenol red is used as indicator.
Other methods: Other methods of concentration are avail-able, which use dilute acids (6% sulfuric acid, 5% oxalic acid, or 3% hydrochloric acid) or mucolytic agents (N-acetyl-L-cysteine along with sodium hydroxide and pancreatin, deso-gen, zephiran, and cetrimide) for concentration of specimens.
◗ Culture
Culture is the definitive method to detect and identify M. tuberculosis. The culture is also more sensitive diagnosticmethod than microscopy of the smear. The culture may be pos-itive with as few as 10–100 AFB per mm of a digested concen-trated specimen. Another advantage of culture is that it helps in specific species identification and for determining drug sus-ceptibility pattern of isolated strains.
LJ medium is an egg-based medium and the Middlebrook 7H10 and 7H11 media are the agar-based media, and these are conventionally used for culture. Since M. tuberculosis is a slow-growing organism, a period of 6–8 weeks is required for colonies to appear on these conventional culture media after incubation at 37°C. Growth of most strains of M. tuberculosis may appear during this period. However, cultures should not be discarded as negative until they have been observed for 12 weeks.
◗ Identification of bacteria
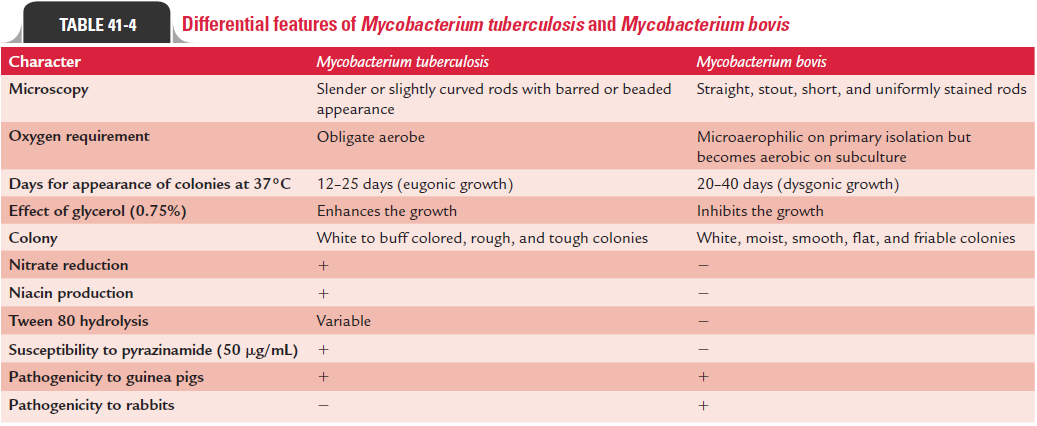
M. tuberculosis organisms are identified by their culture, growth,and biochemical tests. M. tuberculosis is weakly catalase positive, nitrate positive, reduces niacin, and grows slowly over a period of 4–6 weeks. Differences between M. tuberculosis and M. bovis are summarized in Table 41-4.
Niacin test: This test is performed by adding 1 mL of sterilenormal saline to colonies of M. tuberculosis in LJ medium. The medium is kept in a slanting position so that the colonies of mycobacterium are completely covered by normal saline. After sometime, 0.5 mL of the fluid is collected and is placed in a clean screw-capped test tube. To this fluid, 0.5 mL of alcoholic aniline as well as 0.5 mL of 10% cyanogen bromide is added. In a positive test, a yellow color develops in the solution within a few minutes. M. tuberculosis is niacin test negative. It does not produce any yellow color in the fluid.
Arylsulfatase test: This test is performed by culturingmycobacteria in a medium containing 0.001 M tripotassium phenolphthalein disulfate. Mycobacterium species producing the enzyme arylsulfatase split phenolphthalein free from
tripotassium phenolphthalein disulfate. The free phenol-phthalein is detected by adding 2N sodium hydroxide to the culture. Development of pink color indicates positive reac-tion. M. tuberculosis is arylsulfatase negative, while some other Mycobacteriumspecies are arylsulfatase positive.
Neutral red test: This test is carried out to detect ability ofcertain strains of mycobacteria to bind neutral red in an alka-line buffer solution. In this test, one to two colonies of myco-bacteria grown on LJ medium are inoculated with 5 mL of ethyl alcohol collected in a screw-capped bottle. This bottle after inoculation is incubated at 37°C for 1 hour. Supernatant alcohol is then removed carefully and transferred to another bottle to which 5 mL of distilled water and 0.2 mL of 0.05% aqueous solution of neutral red are added. Then N/100 sodium hydroxide is added drop by drop till the color of the fluid becomes amber. The bottle is reincubated at 37°C for 1 hour in water bath with frequent shaking. The development of pink or red stained colonies in the amber-colored fluid sug-gests a positive test. M. tuberculosis and M. bovis are neutral red test positive.
Catalase test: This test is performed to detect the enzymecatalase produced by various Mycobacterium species. This test is performed by mixing equal volumes of 30% hydrogen per-oxide and 10% of Tween 80 in a test tube. Then a few colonies of mycobacteria are picked up by a nichrome wire loop and are inoculated in the fluid. Formation of air bubbles in the fluid is observed within 1 minute. Demonstration of bubbles in the fluid indicates positive test (Fig. 41-3, Color Photo 42). If the bubbles appear in the fluid immediately, it is considered strongly positive, but if appear slowly, it is considered weakly positive. Absence of any bubble is considered as negative test. M. tuberculosis and M. bovis are weakly catalase positive. The bacillilose their catalase activity when they develop resistance to INH.
Amidase test: This test detects the ability of certain mycobac-teria to split amides, such as acetamide, nicotinamide, pyrazin-amide, carbamide, and benzamide. This test is performed by inoculating 0.00164 M solution of the amide with the myco-bacterial culture at 37°C. Then 0.1 mL of manganese sulfate, 1 mL of phenol solution, and 0.5 mL of hypochlorite solution are added to the solution. The tubes are then kept in boiling water bath for 20 minutes. Positive test is indicated by the develop-ment of blue color in the fluid. M. tuberculosis is amidase posi-tive, produces the enzyme nicotinamidase and pyrazinamidase.
Nitrate reduction test: This test detects the presence ofenzyme nitrate reductase produced by M. tuberculosis and otherMycobacterium species. It is performed by adding col-onies of the mycobacteria to buffered solution containing nitrate and incubating at 37°C for 2 hours. After incubation, sulfanilamide and N-naphthyl-ethylene diamine hydrochlo-ride solution is added. Development of pink or red color within 30–60 seconds is suggestive of a positive test. M. tuber-culosis and other mycobacterial species are nitrate reductasetest positive, whereas M. bovis and M. avium are negative.
Tween 80 hydrolysis: This test is performed to demonstratethe enzyme lipase produced by certain mycobacterial species. It is carried out by adding a loopful of mycobacterium culture to a solution containing Tween 80, buffer, and neutral red. The inoculated medium is inactivated at 37°C, and the reaction is observed first at 3–6 hours, then at third day, and thereafter daily up to a period of 10 days. Development of pink color indi-cates positive Tween 80 hydrolysis test. M. tuberculosis shows a variable result. M. kansasii produces a positive test within 3–6 hours, while other mycobacteria species produce positive test within 3–10 days.
NAP differentiation test: This test is based on the principlethat addition of p-nitro acetyl-amino-hydroxyl-propionophe-none (NAP) inhibits the growth of M. tuberculosis as well as M. bovis and M. africanum. This substance does not inhibitgrowth of otherMycobacterium species.
DNA probe: Nucleic acid hybridization using DNA probes isincreasingly used to identify the species. These commercially available probes help in the identification of M. tuberculosis complex much earlier. The DNA probes show a sensitivity and specificity of 100%, when at least 100,000 tubercle bacilli are present. This test uses chemiluminescent ester-labeled single-stranded DNA probes. The chemiluminescence is determined by using luminometer. Positive DNA probe test indicates the mycobacteria to be M. tuberculosis, M. bovis, or M. africanum. The probe cannot differentiate between these species. Niacin pro-duction, nitrate reduction, and production of pyrazinamidases are useful tests to differentiate M. tuberculosis from M. bovis (Table 41-4).

Pyrazinamide test: M. tuberculosisis sensitive to 50mg/mLpyrazinamide and is pyrazinamidase test positive. M. bovis is pyrazinamidase test negative.
TCH susceptibility test: M. tuberculosisis resistant to 10mg/mLof TCH, while M. bovis is susceptible to TCH.
◗ Mycobacteria drug susceptibility testing
With the emergence of multidrug resistant (MDR) myco-bacteria, determination of drug susceptibility testing is impor-tant for starting appropriate treatment. Drug susceptibility can be demonstrated by both (a) phenotypic susceptibility assays and (b) genotypic methods.
· Phenotypic assay methods include (i) resistance ratio method,(ii) absolute concentration method, (iii) proportion method, and (iv) radiometric methods.
· Genotypic methods include (i) DNA sequencing, (ii) solid phase hybridization, and (iii) polymerase chain reac-tion (PCR)—single-stranded combination polymorphism analysis.
◗ Serodiagnosis
M. tuberculosis infection is associated with elevated levels ofantibodies in the serum. Various mycobacterial antigens have been used in these serological tests, which include BCG, 5 and 6 kDa proteins of M. tuberculosis, 32 and 64 kDa protein of M. bovis, etc. Detection of antibodies by serological test, suchas enzyme linked immunosorbent assay (ELISA), is of limited value in the diagnosis of pulmonary tuberculosis. Latex agglu-tination test using latex particles coated with rabbit antibody against M. tuberculosis has been used for demonstration of anti-gen in the CSF for diagnosis of tuberculosis meningitis.
◗ Animal inoculation
Guinea pig inoculation is carried out by intramuscular inocu-lation of concentrated clinical specimens into thighs of two 12 weeks’ old healthy guinea pigs. Development of infection is suggested by a progressive loss of weight and development of a positive tuberculin skin reaction. One of the two animals is sacrificed after 4 weeks.
In a positive infection, the autopsy of the animal shows a caseous lesion at the site of inoculation. At times, the local cutaneous lesion may be associated with formation of pus at the site of the cutaneous lesion and enlarged caseous lymph nodes draining the region. Spleen, liver, peritoneum, and lungs show development of tubercles varying 1–2 mm in diameter. The stained smear of the exudates shows the presence of AFB by microscope.M. tuberculosis is highly pathogenic for guinea pigs, while catalase-negative INH-resistant strains and most strains isolated from south India are low pathogenic for guinea pigs. M. tuberculosis is nonpathogenic for rabbits. M. bovis is pathogenic for both rabbits and guinea pigs.
Inoculation in guinea pigs was earlier widely used for diagno-sis of tuberculosis. But guinea pig inoculation is now regarded as obsolete, because it is cumbersome, costly, and less sensitive than culture. Guinea pig inoculation is, however, superior to culture for isolation of M. bovis from clinical samples.
◗ Tuberculin skin test
Tuberculin skin test (TST) is a widely used test for diagnosis of tuberculosis:
· It is used for evaluation of cases that have tuberculosis and for diagnosis of cases suspected to be infected withM. tuberculosis.
· It is used to diagnose active tuberculosis infection in infants and young children.
· It is also used to select population susceptible for BCG (bacille Calmette–Guerin) vaccination.
· It is a valuable test to measure prevalence of tuberculosis infection in a community.
TST can be performed by the following methods:
Mantoux test: Mantoux test is the recommended methodof skin test. The test is performed by intradermal injection of 0.1 mL or 5 tuberculin units (TU) PPD into the volar aspect of the forearm using a 27-G needle. It is essential that PPD is injected between the layers of the skin, but not subcutane-ously. Development of an induration of 10 mm or more at the site of injection after 48–72 hours is considered a positive test. Induration less than 5 mm is negative, while between 6 and 9 mm is considered equivocal. Erythema is not considered in reading of the test.
If the test is negative with the PPD of 5 TU, then the test may be repeated using PPD of 10 or 100 TU. Purified protein derivative of 1 TU is used when the recipient is considered to be extremely hypersensitive to the antigen.
Multiple puncture tests: These include the Heaf test andtime test. Heaf test is performed by using the hit gun and by using disposable prongs carrying dried PPD. These tests may be satisfactory for screening and surveys but are not useful as diagnostic test because they lack sensitivity and specificity.
A positive skin test indicates hypersensitivity of the indi-vidual to tubercle protein. This suggests infection with M. tuberculosis or immunization by BCG vaccination in recentor past with or without clinical disease. The TST becomes posi-tive 4–6 weeks after infection or immunization. The tubercle test positivity gradually disappears within 4–5 years in absence of reexposure to tubercle bacilli. In endemic areas, this positiv-ity is maintained due to repeated exposure to tubercle bacilli. A negative tubercle test indicates that the person has never come in contact with tubercle bacilli.
The TST may show false-positive and false-negative reactions:
1. False-positive reaction occurs in patients with infection byenvironmental nontuberculous mycobacteria.
2. False-negative reactions may occur in patients with (a) vacci-nation, (b) immunosuppressive therapy, (c) impaired CMI, (d) lymphoreticular malignancies, (e) immunodeficiency, (f) malnutrition, and (g) miliary tuberculosis. Improper administration, such as subcutaneous injection of PPD or injection of too little volume of antigen, contamination of PPD, or improper storage are the other factors that may give rise to false-negative reactions.
◗ Rapid and automated methods
The conventional methods are very slow and time consuming and require 6–8 weeks for isolation of M. tuberculosis. Hence, recently more rapid and automated methods are being increas-ingly used for diagnosis of tuberculosis. These recent meth-ods include automated radiometric culture methods (e.g., BACTEC), SEPTICHEK, mycobacterial growth indicator tubes (MGITs), etc.
· Automated radiometric culture methods, such as BACTEC employaliquidMiddlebrook7H12mediumcontainingradio-metric palmitic acid labeled with radioactive carbon-14 (14C). The medium also contains several antimicrobial agents to prevent the growth of other nonmycobacterial microbes. The result of the test is noted by demonstration of radiola-beled 14CO2 produced during the growth of mycobacteria. Growth of mycobacteria is usually detected within 9–16 days.
· SEPTICHEK is another rapid method for isolation of myco-bacteria. This is a nonradiometric method, which is based on a biphasic broth-based system that decreases the mean recovery time versus conventional methods.
· A new method employs MGITs, which show microbial growth and provide a quantitative index of M. tuberculosis growth. Round-bottom tubes with oxygen-sensitive sensors at the bottom are used in the test.

Related Topics