Chapter: Medical Electronics : Radiological Equipments
Ionising Radiation
IONISING RADIATION
In
general, ionizing radiation is harmful and potentially lethal to living beings
but can have health benefits in radiation therapy for the treatment of cancer
and thyrotoxicosis.
Most
adverse health effects of radiation exposure may be grouped in two general
categories:
·
Deterministic effects (harmful tissue reactions)
due in large part to the killing/ malfunction of cells following high doses;
and
·
Stochastic effects, i.e., cancer and heritable
effects involving either cancer development in exposed individuals owing to
mutation of somatic cells or heritable disease in their offspring owing to
mutation of reproductive (germ) cells.
Its most
common impact is the stochastic induction of cancer with a latent period of
years or decades after exposure. The mechanism by which this occurs is well
understood, but quantitative models predicting the level of risk remain
controversial. The most widely accepted model posits that the incidence of
cancers due to ionizing radiation increases linearly with effective radiation
dose at a rate of 5.5% per sievert. If this linear model is correct, then
natural background radiation is the most hazardous source of radiation to
general public health, followed by medical imaging as a close second. Other
stochastic effects of ionizing radiation are teratogenesis, cognitive decline,
and heart disease.
High
radiation dose gives rise to Deterministic effects which reliably occur above a
threshold, and their severity increases with dose. Deterministic effects are
not necessarily more or less serious than stochastic effects; either can
ultimately lead to a temporary nuisance or a fatality. Examples are: radiation
burns, and/or rapid fatality through acute radiation syndrome, chronic
radiation syndrome, and radiation-induced thyroiditis.
Beneficially,
controlled doses are used for medical imaging and radiotherapy, and some
scientists suspect that low doses may have a mild hormetic effect that can
improve health, but the US National Academy of Sciences Biological Effects of
Ionizing Radiation Committee "has concluded that there is no compelling
evidence to indicate a dose threshold below which the risk of tumor induction
is zero
When
alpha particle emitting isotopes are ingested, they are far more dangerous than
their half-life or decay rate would suggest. This is due to the high relative
biological effectiveness of alpha radiation to cause biological damage after
alpha-emitting radioisotopes enter living cells. Ingested alpha emitter
radioisotopes such as transuranics or actinides are an average of about 20
times more dangerous, and in some experiments up to 1000 times more dangerous
than an equivalent activity of beta emitting or gamma emitting radioisotopes.
·
Medical use of rad iation based on the fact that
that it can dest roy cells (instrument sterilisation, treatm ent of cancer)
·
Medical use of radiation based on the fact that
radiation is easy to detect
Types of ionizing radiation
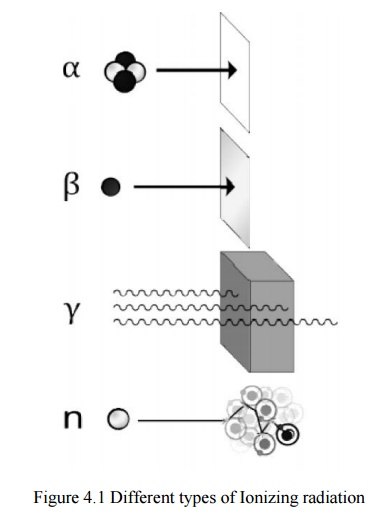
Alpha (α) radiation cons ists of a fast-moving
helium-4 (4He) nucleus and is stopped by a sheet of paper. Beta (β) radiation, consisting of electrons,
is halted by an aluminium plate. Gamma (γ)
radiation, consistingg of energetic photons, is eventually absorbed as it
penetrates a dense material. Neutron (n)
radiation consists of free neutrons that are blocked using light elements, like
hydrogen, which slow and/or capture them. Not shown: gala ctic cosmic rays that
consist of energetic charged nu clei like protons, helium nuclei, and
high-charged nuclei called HZE ions.
Ionizing
radiation is cate gorized by the nature of the particles or ele ctromagnetic
waves creating the ionising effect. The se have different ionization
mechanisms, an d may be grouped as directly or indirectly ionizing.
Directly ionizing
Any
charged massive particle can ionize atoms directly by funddamental interaction
through the Coulomb force if it carries sufficient kinetic energy. This
includes atomic nuclei, electrons, muons, charged pions, protons, and energetic
charged nuclei stripped of their electrons, all of which must be moving at
relativistic speeds to reach the required kinetic energy. The first two to be
recognized were given special names, which are used today: Helium nuclei at
relativistic speeds are called alpha particles, and electrons at relativistic
speeds are called beta particles. Natural cosmic rays are made up primarily of
relativistic protons but also include heavier atomic nuclei like helium ions
and HZE ions and muons. Charged pions are very short-lived and seen only in
large amounts in particle accelerators.
Alpha particles
Alpha
particles consist of two protons and two neutrons bound together into a
particle identical to a helium nucleus. They are generally produced in the
process of alpha decay, but may
also be
produced in other ways. Alpha particles are named after the first letter in the
Greek alphabet, α. The symbol for the alpha particle is α or α2+.
Because they are identical to helium
nuclei,
theyarealsosometimeswrittenasHe2+or 42He2+indicating a Helium ion with a +2
charge (missing its two electrons). If the ion gains electrons from its
environment, the alpha particle can be written as a normal (electrically
neutral) Helium atom 42He.
They are
a highly ionizing form of particle radiation, and when resulting from
radioactive alpha decay have low penetration depth. They can be stopped by a
few centimetres of air, or by the skin. However, so-called long range alpha
particles from ternary fission are three times as energetic, and penetrate
three times as far. The helium nuclei that form 10-12% of cosmic rays are also
usually of much higher energy than those produced by nuclear decay processes,
and are thus capable of being highly penetrating and able to traverse the human
body and dense shielding, depending on their energy.
Beta particles
Beta
particles are high-energy, high-speed electrons or positrons emitted by certain
types
of
radioactive nuclei, such as potassium-40. The production of beta particles is
termed beta decay. They are designated by the Greek letter beta (β). There are
two forms of beta decay, β−
and β+,
which respectively give rise to the electron and the positron.
High-energy
beta particles may produce X-rays known as bremsstrahlung ("braking
radiation") or secondary electrons (delta ray)as they pass through matter.
Both of these can subsequently ionize as an indirect ionization effect.
Bremsstrahlung
is of concern when shielding beta emitters, because interaction of beta
particles with the shielding material produces Bremsstrahlung radiation. This
effect is greater with material of high atomic numbers, so material with low
atomic numbers is used for beta source shielding.
Positrons
The positron or ant electron is the antiparticle or the antimatter counterpart of
the electron. The positron has an electric charge of +1e, a spin of ½, and has
the same mass as an electron. When a low-energy positron collides with a
low-energy electron, annihilation occurs, resulting in the production of two or
more gamma ray photons (see electron–positron annihilation).
Positrons
may be generated by positron emission radioactive decay (through weak
interactions), or by pair production from a sufficiently energetic photon.
Positrons are common artificial sources of ionizing radiation in medical PET
scans.As positrons are positiv ely charged particles they can also directly
ionize an atom through Coulomb interactions.
Indirectly ionizing
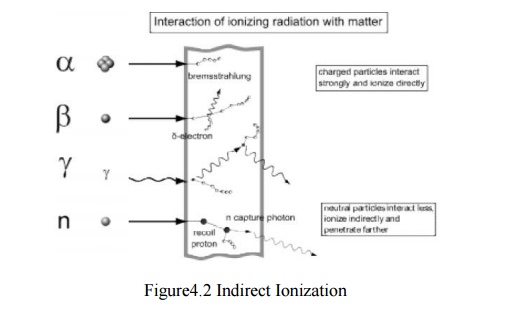
Radiation
interaction - gamma rays are represented by wavy lines, charged particles and
neutrons by straight lines. The small circles show where ionization occ
urs.Indirect ionizing radiation is electrically neutral a nd therefore does not
interact strongly with matter. The bulk of the ionization effects are due to
secondary ionizations.
3 main uses of ionising radiatio n in medicine:
•
Treatment
•
Diagnosis
•
Sterilisation
Cancer
Cancers
are growths of cells (cancerous tumours) which are out of co ntrol. As a result
of this, they do not perform their intended function.
Treatment of Cancer
Cancerous
tumours can be treated using the
following main methods:
•
Chemotherapy (drugs).
•
Radiation therapy (radi otherapy and
brachytherapy).
Surgery.
Factors
which affect the choice of treatment for cancer. The choice of treatment depends
on a number of factors including:
•
The size of the tumour.
•
The position of the tumo ur.
The Aims of Radiation Therapy
The aim
of radiation therapy is to cause damage to the cancerous cells whilst
minimising the risk to surrounding healthy tissue
The damage
inflicted by radiation therapy causes the cancerous cells to stop reproducing
and thus the tumour shrinks. Unfortunately, healthy cells can also be damaged
by the radiation. The amount of radiation given to the patient has to be
accurately calculated so that the damage is limited to the cancerous cells
only.
Radiation Therapy
Radiation
therapy uses ionising radiation to treat cancer i.e. to destroy cancerous
cells. There are two techniques in radiation therapy that are used to treat
cancer using ionising radiation:
Diagnosis
Static Imaging
•
There is a time delay between injecting the tracer
and the build-up of radiation in the organ. Static studies are performed on the
brain, bone or lungs scans
Dynamic Imaging
•
The amount of radioactive build-up is measured over
time.
•
Dynamic studies are performed on the kidneys and
heart.
•
Renograms are dynamic images of the kidneys and
they are performed for the following reasons:
•
To assess individual kidney and/or bladder
function.
•
To detect urinary tract infections.
•
To detect and assess obstructed kidney(s).
•
To detect and assess vesico-ureteric reflux.
•
To assess kidney transplant(s).
Sterilisation
•
Radiation not only kills cells, it can also kill
germs or bacteria.
•
Nowadays, medical instruments (e.g. syringes) are
prepacked and then irradiation using an intense gamma ray source.
•
This kills any germs or bacteria but does not
damage the syringe, nor make it radioactive.
Related Topics