Chapter: Medical Immunology: Transplantation Immunology
Immunosuppression - Transplantation Immunology
IMMUNOSUPPRESSION
The ideal transplantation should take place between genetically identical individuals. This is only possible in the rare event of transplantation between identical twins. Thus, the suc-cess of clinical transplantation depends heavily on the use of nonspecific immunosuppres-sive agents that, by decreasing the magnitude of immunological rejection responses, pro-long graft survival. Current immunosuppression in transplanted patients is achieved by the use of cytotoxic/immunosuppressant drugs and biological response modifiers, such as an-tilymphocyte antibodies.
A. Chemical Immunosuppression
Several drugs are currently used to induce immunosuppression including glucocorticoids, antimetabolites, cyclosporin A and tacrolimus (FK507).
1. Glucocorticoids
These are used to treat and prevent rejection. They have multiple effects on the immune system, including lymphocyte apoptosis, inhibition of antigen-driven T-lymphocyte prolif-eration, inhibition of IL-1 and IL-2 release, and inhibition of chemotaxis. Because of the side effects associated with the use of glucocorticoids in relatively large doses for long pe-riods of time (as required in transplantation), they are usually administered together with other immunosuppressant drugs, allowing the reduction of steroid doses below levels caus-ing major side effects.
2. Antimetabolites
These are mostly used in the prevention of rejection episodes. All these agents inhibit DNA replication, lymphocyte proliferation, and the expansion of antigen-reactive clones of lym-phocytes.
Azathioprine (Imuran) undergoes metabolic conversion into 6-mercaptopurine, which inhibits purine nucleotide synthesis and prevents lymphocyte proliferation (both T and B). Mycophenolate mofetil (CellCept) is converted to mycophenolic acid, which is an inhibitor of inosine monophosphate dehydrogenase, a participant in guanosine nucleotide synthesis. This agent has virtually replaced azathioprine as a maintenance immunosup-pressant. Cyclophosphamide (Cytoxan) is an alkylating agent that modifies DNA and pre-vents lymphocyte replication. This drug is rarely used in solid organ transplantation.
3. Calcineurin Inhibitors
Cyclosporin A (Sandimmune, Neoral) is used in the prevention and treatment of rejection. Its introduction in 1983 had a marked impact on the survival of transplanted organs, which increased by at least 20–30% in the case of kidney and heart grafts. The revival of interest in heart transplants in the last two decades was a direct consequence of the availability of cyclosporin A (CsA), and the success of liver transplants is also directly related to the use of this drug. CsA is particularly helpful in the prevention of rejection, usually administered in association with glucocorticoids, because of its steroid-sparing effect.
The effects of CsA are mainly related to the inhibition of activity of transcriptional activators controlling the expression of IL-2 and other lymphokine genes in helper T cells, thus curtailing the onset of both cellular and humoral immune responses.
CsA itself has marked toxicity. It is nephrotoxic (problematic in patients receiving kidney transplants, in which it will be necessary to differentiate between acute rejection and CsA toxicity) and causes hypertension. Monitoring of circulating cyclosporine levels is es-sential to minimize the toxic effects of this drug.
Tacrolimus (Prograf, FK506) has a mechanism of action similar to CsA, but it is able to reverse rejection episodes in patients unresponsive to other immunosuppressive agents. Tacrolimus is used in a fashion similar to cyclosporine in combination with glucocorticoids and antimetabolites. It also has toxic effects including nephrotoxicity and neurotoxicity.
4. Rapamycin (Sirolimus, Rapamune)
This is a unique compound that is structurally similar to Prograf, but its mechanism of ac-tion seems to be related to cell cycle inhibition. It is currently occasionally used instead of Cellcept or Imuran.
B. Biological Response Modifiers
This group includes a variety of biological compounds that have been found to be useful in the prevention and treatment of graft rejection.
1. Antithymocyte and antilymphocyte Globulins
These were among the earliest successful therapeutic agents used in the management of graft rejection. These reagents are gammaglobulin fractions separated from the sera of an-imals (rabbits, goats, or horses) injected with human thymic lymphocytes or human pe-ripheral blood lymphocytes. They are very effective in the prevention and reversal of re-jection episodes, and their mechanism of action is related to the destruction or inhibition of recipient lymphocytes. Their main drawbacks have been related to difficulty in obtaining standardized preparations, reactivity with other cell types, and frequent sensitization of the patients, which often leads to serum sickness when the globulins are administered repeatedly.
2. Anti–T-Cell Monoclonal Antibodies
These antibodies, derived from mouse B cells and directed against human T cells, particu-larly those reacting with the CD3 marker (OKT3), have been extensively used in the man-agement of transplanted patients. Their mechanism of action is not entirely clear. OKT3 has been reported to cause depletion of CD3+ T lymphocytes, and it is likely that the depletion is due to complement-mediated lysis, opsonization, and ADCC. OKT3 also causes down-modulation of CD3 on the cell surface of otherwise viable T cells and may induce T-cell anergy.
These antibodies are predominantly used for the treatment of acute rejection. In ad-dition, some groups use the monoclonal antibody as “induction” treatment at the time of transplantation to prevent rejection. As with antilymphocyte and antithymocyte globulins, the possibility of using monoclonal antibodies to treat repeated episodes of rejection is lim-ited by the sensitization of the patients receiving the antibody. It must be noted that in spite of concomitant immunosuppression, up to 30% of patients become sensitized to various an-tibody preparations. However, changing to a different monoclonal antibody can meet with success in hypersensitive patients.
Besides serum sickness, monoclonal and polyclonal antibodies can cause what is known as the cytokine syndrome, which presents with fever, chills, headaches, vomiting, diarrhea, muscle cramps, and vascular leakage and vascular transudation. Experiments and data suggest that the syndrome is caused by massive interleukin release from T cells acti-vated as a consequence of the binding of these antibodies to the lymphocyte membrane.
Both monoclonal and polyclonal antilymphocyte antibody preparations are strongly immunosuppressive, so the risk of developing life-threatening infections or non-Hodgkin’s lymphomas is markedly higher in patients treated with them . As a result, treat-ment with these agents usually does not exceed 14 days, and repeated courses of antibody treatment are usually contraindicated.
3. Other Monoclonal Antibodies
Anti-IL-2R monoclonal antibodies (Daclizumab, Simulect, Zenapax) are also approved for use in transplantation for the prevention of rejection. A study published in early 2000 re-ported that induction therapy with one of these antibodies prior to heart transplantation re-duced the frequency and severity of rejection.
AntiCD154 monoclonal antibodies have been used successfully to prevent rejection of kidney allografts in nonhuman primates. The animals received humanized antibodies for up to 5 months. No additional therapy was given, and the grafts were not rejected, even af-ter discontinuation of the monoclonal antibody. These experimental studies are certainly encouraging and may open the door to human protocols.
The problem of sensitization can be minimized when genetically engineered mono-clonal antibodies containing the binding site of a murine monoclonal antibody and the con-stant regions of a human immunoglobulin are used. These “humanized” or chimeric mon-oclonals can be administered for more prolonged periods of time without sensitization occurring. However, they are considerably more expensive.
C. Total Lymphoid Irradiation
Irradiation of those areas of the body where the lymphoid tissues are concentrated is almost exclusively used to prepare leukemic patients for bone marrow transplantation. In this cir-cumstance, irradiation combines two potential benefits: the elimination of malignant cells and the ablation of the immune system. Another immunosuppressive effect of irradiation is due to the greater radiosensitivity of helper T cells, resulting in the survival and predomi-nance of suppressor/cytotoxic T cells among the residual lymphocyte population after ir-radiation.
Transplantation preconditioning protocols with total lymphoid irradiation have met with some impressive success: the patients are reported to require very low doses of main-tenance immunosuppressive drug, and in a few cases it has been possible to withdraw the immunosuppressive drugs completely.
D. The Transfusion Effect: Stem Cells and Microchimerism
Although blood transfusions were generally avoided in potential transplant recipients due to the fear of sensitization to HLA, blood group, and other antigens, several groups reported in the early 1980s that kidney graft survival was longer in patients who had received blood prior to transplantation (Fig. 25.2). This led to attempts to precondition transplant recipi-ents with multiple pretransplant transfusions. Several interesting observations were recorded during these attempts.
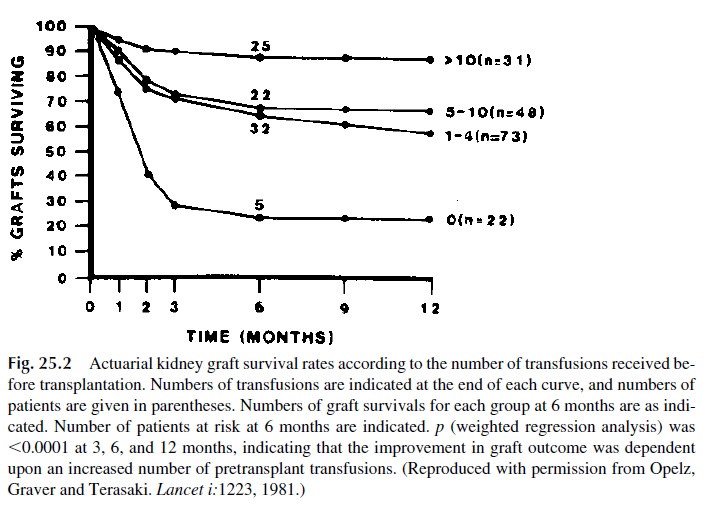
1. The effect is more pronounced if MHC-II–expressing cells are included in the transfusion. Thus, the administration of whole blood, packed cells, or buffy coat is more efficient than the administration of washed red cells in improving graft survival.
2. The protection can be induced with a few donor-specific transfusions but usually requires multiple random transfusions. This probably reflects a MHC-specific effect that is obviously easier to achieve when the transfused cells have the same MHC as the graft.
3. The transfusion effect is delayed—usually seen about 2 weeks after a donor-spe-cific transfusion. Following transfusion there is a depression of cellular immu-nity, which, according to some studies, seems to become more accentuated and longlasting with repeated transfusions.
The concept that emerged from these studies was that the transfusion protocols in-duce a state of at least partial tolerance to the graft. Among the several mechanisms pro-posed to explain the transfusion effect, three deserve special mention:
1. According to the some authors, pretransplant transfusion would sensitize the pa-tient against MHC antigens of the transplanted tissue. In some patients these are cytotoxic antibodies that preclude transplantation.
In others, the MHC antibod-ies could function as enhancing antibodies by two mechanisms: (1) blocking the immunogenic sites on MHC molecules, thus inhibiting the initiation of the re-jection reaction, and (2) binding to complexes of cells expressing the nonself al-loantigen and host cells recognizing alloantigen and promoting the phagocytosis and destruction of the cellular complex via Fc-mediated opsonization
2. Other groups suggest that the administration of blood transfusions may also transfer donor-derived hematopoietic stem cell precursors. This could lead to the establishment of a low level of donor-derived cells within the recipient bone marrow and peripheral blood and result in a state of microchimerism. The mi-crochimeric state could then induce tolerance to the donor tissues and, conse-quently, result in improved graft survival. The induction of microchimerism, however, can be more efficiently achieved by partial ablation of the host immune system followed by transplant of the donor’s bone marrow transplantation. How-ever, at this time is not clear whether such aggressive protocols have any practi-cal advantages over the simple administration of donor hematopoietic cells.
3. Another possible mechanism leading to a state of unresponsiveness to the MHC antigens of a graft is a lack of delivery of co-stimulatory signals to the respond-ing cells of the recipient. This scenario is facilitated by the fact that the patients receive high doses of immunosuppressive agents. The lack of co-stimulation of presensitized T lymphocytes responding to the grafted tissue could result in apoptosis of the responding cells, i.e., in the deletion of clones reactive against the grafted tissue.
Pretransplant transfusions have been very much abandoned, because with improve-ments in matching and immunosuppression protocols, the transfusion effect became less and less evident. However, there is considerable interest in developing protocols using stem cell administration as a way to induce a more complete tolerance state that could allow sus-pension or significant dose reduction of immunosuppressive drugs.
E. Immunosuppression Side Effects
Effective long-term immunosuppression is inevitably associated with a state of immunoin-competence. Two major types of complications may result from this.
1. Opportunistic Infections
The immunosuppressed patient is susceptible to a wide variety of infections, particularly caused by infectious agents that are not often seen as pathogens in immunocompetent in-dividuals, such as cytomegalovirus, herpesviruses (Epstein-Barr virus, herpes simplex virus, varicella-zoster virus), Pneumocystis carinii, Toxoplasma gondii, and fungi (e.g., Candida albicans). Cytomegalovirus (CMV) infections are particularly ominous becausethis virus can further interfere with the host’s immune competence and may also trigger re-jection in a nonspecific way.
The incidence of infections in transplant patients can be reduced by prophylactic therapy with intravenous gammaglobulin, which is part of most post–bone marrow trans-plant protocols, since those patients are probably the most profoundly immunosuppressed. Bone marrow and solid organ transplant patients also usually receive prophylactic antibi-otics such as trimethoprim-sulfamethoxazole (for Pneumocystis,urinary tract infections, pneumonia, and cholangitis prophylaxis), ganciclovir or acyclovir (for herpes and CMV prophylaxis), and clotrimazole or fluconazole (for Candida prophylaxis).
2. Malignancies
Either as a consequence of the oncogenic properties of some immunosuppressive agents or as a consequence of disturbed immunosurveillance, the incidence of malignancies is sig-nificantly increased in transplant patients. In patients with survival times following trans-plantation of 10 years or longer, the frequency of skin cancer (squamous or basal cell car-cinoma) may be up to 40%, although the lesions are no more invasive than in normal individuals. An additional 10% may develop other types of malignancies, including EBV-associated, posttransplant lymphoproliferative disorder (PTLD).
The reasons for the predominance of skin cancer and PTLD among transplant pa-tients may relate to the inability of the immune system to respond to papillomaviruses and Epstein-Barr virus (EBV), which are etiological agents for these malignancies, respec-tively. Some of these PTLD are reversible with interruption or reduction of immunosup-pressive therapy, while others are true malignant lymphomas that may spread to areas usu-ally spared in nontransplanted patients, such as the brain.
Common cancers such as colon, lung, and breast are seen no more frequently in trans-plant patients than in the normal population. This suggests that immune surveillance is not important for most common cancers.
3. Other Side Effects
The most widely used immunosuppressive agents have specific side effects that may have a significant negative impact on the quality of life of transplanted patients. Glucocorticoids can cause, among other side effects, obesity, insulin-resistant diabetes, cataracts, avascular necrosis of the femoral head, and thinning of the skin. Antimetabolites are associated with decreased blood counts and bone marrow depression. Cyclosporine and tacrolimus cause hypertension, nephrotoxicity, and neurotoxicity.
The use of combinations of different immunosuppressive drugs usually reduced the incidence and degree of side effects, because each drug that is part of the combination can be used at relatively well-tolerated doses. However, the ultimate goal of transplanta-tion researchers is to develop protocols that would not require maintenance immunosup-pression.
Related Topics