Chapter: Biochemistry: Biological Oxidation
High energy compounds
High energy compounds
The high energy compound is the ATP. The other
high energy compoundsinclude ADP,1,3-diphospho glycerate, phosphoenol pyruvate
and also creatine phosphate.
The phosphate group of the high energy phosphate
may transfer directly to another organic compound. For this reason the term
group transfer potential is preferred by some high energy bond. However, the
phosphorylated compound may or may not have high energy phosphate bond, though
the total energy content of the molecule is higher than a non phosphorylated
compound.
Storage form of high energy compounds
They are called as phosphogens and help to store the high energy. The example for this
the creatine phosphate present in the vertebrate muscles, the reaction works in
both directions it is a reversible reaction form ATP when ATP is required. When
ATP is more, creatine reacts with ATP and forms the phosphocreatine.
1. 1, 3-diphosphoglycerate
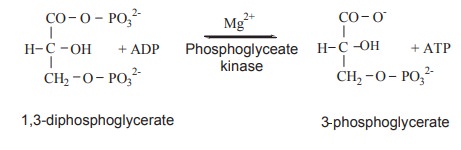
One of the phosphate groups undergoes hydrolysis
to form the acid and a phosphate ion, giving off energy. This first energy
producing reaction is coupled with the next endothermic reaction making ATP.
The phosphate is transferred directly to an ADP to make ATP and this is
catalysed by phosphoglycerate Kinase enzyme. Since one molecule of glucose
yield 2 molecule of Glyceraldehyde 3-phosphate, 2 high energy ATP are produced
for one molecule of glucose.
Role of Phosphoenol pyruvate
Phosphoenol pyruvate, which is formed during
breakdown of glucose to lactic acid, donates its phosphate group to ADP in a
reaction catalyzed by Pyruvate kinase. One of the phosphate groups undergoes
hydrolysis to form the acid and a phosphate ion, giving off energy. This first
energy producing reaction is coupled with the next endothermic reaction making
ATP. The phosphate can only exist as the high energy enol form. Thus, when the
phosphate group is removed, the pyruvate can revert back to the stable,
low-energy keto form and the surplus energy is released. Production of ATP in
this reaction is controlled by pyruvate kinase
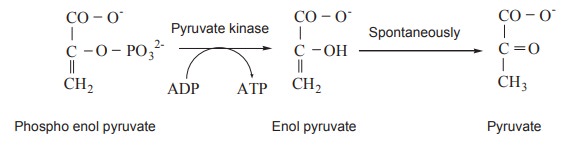
Actually, this reaction takes place in two
steps. First the enolate form of pyruvate is formed, then the transfer of the
phosphate group to ADP occur as second step. The keto pyruvic acid may reduced
to lactic acid in the lack of oxygen. Mitochondria isnot involved . Since
one molecule of glucose yield 2 molecule of Glyceraldehyde 3phosphate, 2 high
energy ATP are produced for one molecule of glucose.
Phosphocreatine
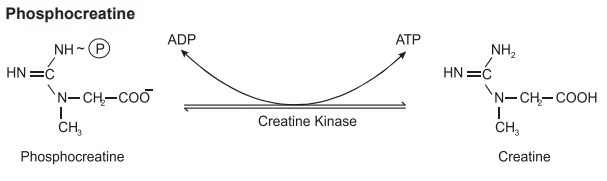
Phosphocreatine is a phosphogen and it interacts
with ADP to form ATP. When ATP is more creatine reacts with ATP and forms
phosphocreatine. The enzyme involved is creatine kinase. This energy transfer
from creatine phosphate to ADP helps to produce ATP molecule to provide energy
during muscle contraction.
2. ATP as high energy compound
ATP is the most widely distributed high-energy
compound within the human body. Adenosine triphosphate (ATP) is a useful
free-energy currency because the dephosphorylation reaction or hydrolysis,
yield an unusually large amount of energy; i.e., it releases a large amount of
free energy. “High energy” bonds are often represented by the “~” symbol
(squiggle), with~P representing a
phosphate group with a high free energy on hydrolysis. The terminal phosphate
group is then transferred by hydrolysis to another compound, a process called phosphorylation, producing ADP,
phosphorylated new compound and energy.Thus,the
dephosphorylation reaction of ATP to ADP and inorganic phosphate is often
coupled with non spontaneous reactions. Generally, ATP is connected to another
reaction—a process called coupling
which means the two reactions occur at the same time and at the same place,
usually utilizing the same enzyme complex. Release of phosphate from ATP is
exothermic (a reaction that gives off heat) and this reaction is connected to
an endothermic reaction (requires energy input in order to occur). The free
energy yielded can be coupled to endothermic reaction and useful for the works
such as:
Chemical
work: ATP energy is
consumed to synthesize macromolecules that makeup the cell.
Transport
work: ATP energy is
utilized to pump substances across the plasmamembrane.
Mechanical
work: ATP provides energy
to contract the muscles of the body.
Some time the phosphate group can be transferred
to an acceptor molecule and such group transfer potential are associated with
some high energy compound. Thus, ATP act as a common intermediate that serves
as a vehicle for transfer of chemical energy.
2.1. Structure of ATP
ATP is an abbreviation for adenosine triphosphate, a complex molecule that contains the
nucleoside adenosine, ribose and a
tail consisting of three phosphates.
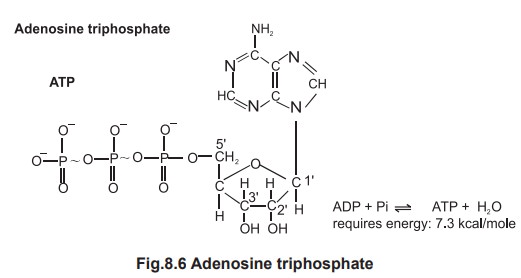
The bond is known as a “high-energy” bond and is
depicted in the figure 8.6 by a wavy line. The bond between the first and the
second phosphate is also “high-energy” bond.
2.2 Free energy of hydrolysis of ATP

ATP is sometimes referred to as a “High Energy”
compound. High energy in this case does not
refer to total energy in compound, rather just to energy of hydrolysis. Thus
ATP has a larger negative DG for hydrolysis. For biochemistry HighEnergy is defined in terms of ATP:
if a compound’s free energy for hydrolysis isequal to or greater than ATP’s
then it is “High Energy,” if its free energy of hydrolysis is less than ATP’s
then it is not a “high energy” compound. Note that ATP has two high energy
anhydride bonds (AMP ~P~P). DG of
ATP hydrolysis also depends on the local environments it varies with pH,
divalent metal ion concentration, ionic strength and Consumption of ATP. An EATP
of -7.3 kcal /mol requires ATP, ADP and phosphate to be present at equal
concentrations. In cells, however the concentration of ATP is often 5 to 10
times that of ADP.
As a result, the free energy of ATP hydrolysis
is about -12 kcal / mol. One must be clear that the bond energy generally meant
by physical chemist is the energy required to break a covalent bond between two
atoms. Since relatively a large amount of energy is required to break a
covalent bond, the phosphate bond energy is totally a different one. Phosphate
bond energy specifically denotes the difference in the free energy of the
reactants when phosphorylated compound undergoes hydrolysis.
2.3 Mono (ortho) phosphate cleavage and Pyrophosphate cleavage
ATP may under go either an orthophosphate or
pyrophosphate cleavage during it’s utilization in biosynthetic pathways. In an
ATP molecule, when the terminal phosphate is cleaved it is called as mono
phosphate or ortho phosphate cleavage.
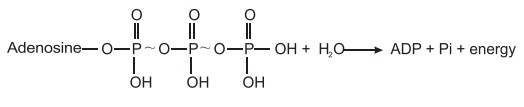
However, in many ATP utilizing reactions instead
of one terminal phosphate two terminal phosphate groups are enzymatically
hydrolyzed to give a pyro phosphate molecule and a large amount of energy which
is greater than the mono phosphate or ortho phosphate cleavage.
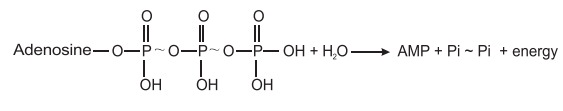
Pyrophosphate
(PPi) is often the product
of a reaction that needs a drivingforce. Its spontaneous hydrolysis, catalyzed
by Pyrophosphatase enzyme, drives the reaction for which PPi is a substrate.
The DG (free energy) for this pyrophosphate cleavage is 10.0 Kcal./mol and thus
an extra thermodynamic push is given to certain enzymatic reaction which
require more energy than that of a mono phosphate cleavage and assure the completeness
of certain biosynthetic reactions
Related Topics