Chapter: Biochemistry: Biological Oxidation
Electron transport chain
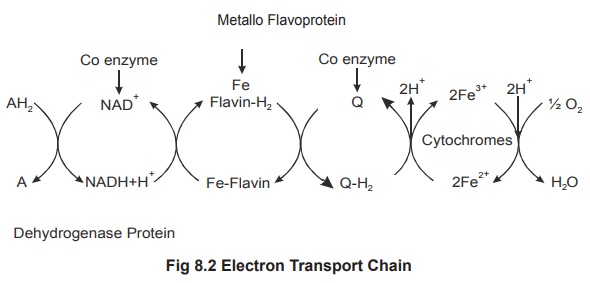
Electron transport chain
The electron transport chain (figure 8.2)
consists of series of proteins which tightly bound involves the passage of a
pair of electrons from one chemical to the next, whereby each chemical in the
sequence has less reduced energy than the previous. The electron transport
chain oxidizes (i.e. “burns”) the NADH+H+ and FADH2 cofactors, using
molecular oxygen as the final electron acceptor. In the electron transport
chain electron carriers and hydrogen-electron acceptors are positioned
alternatively to carry the function. There are three different regions in the
electron transport chain, where energy is released. In each region there is a
formation of one ATP. All these reactions and capturing of energy takes place
in mitochondria.
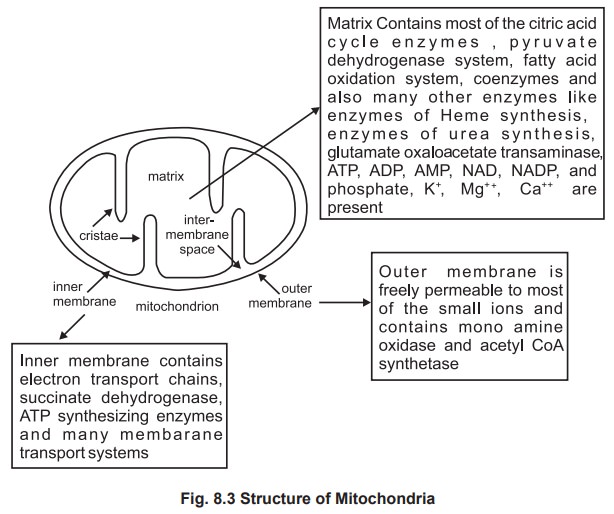
1. Components of mitochondria with marker enzymes
The histochemical and ultra centrifugation
studies clearly established that the major site of cellular oxidation is
mitochondria. These are sub cellular organelles and quite vary in size and
shape. Ellipsoidal, spherical or rod shaped structures measuring about 0.5-5 μ
in length and 0.1-0.6μ in width. Since, the energy released in the oxidation
process is converted into chemical energy (ATP). Mitochondria otherwise called as
power house of the cell. Hence the
number of mitochondria in a cell depends onit’s metabolic activity. All the
reducing equivalence that can release energy during oxidation of carbohydrates,
fatty acids and proteins are available in the mitochondria. In mitochondria, a
series of catalysts referred as respiratory chain that collects these reducing
equivalents and direct them towards oxygen to form water.
The electron microscopic picture of the
mitochondria shows a double membrane, an outer and inner membrane which
consists of different specific enzymes. The folding of the inner membrane
produces a number of partitions called cristae that extend into the matrix.
The inner membrane encloses the matrix and itis very selective in its
permeability. Inner membrane is highly complex in its structure and function.
The space between the inner and the outer membrane is called as Inter
membrane space which is surrounded bymatrix. The mitochondria containsits own circular DNA and
ribosomes. Some mitochondrial proteins are thus coded for and produced by the
mitochondria itself. Other mitochondrial proteins are coded by nuclear DNA,
synthesized by cytosolic ribosomes, and subsequently transported to the
mitochondria.
The structure of mitochondria ( figure 8.3 ) and
the location of various essential enzymes are given in the form of diagram.
Since these enzymes
2. Members of the electron transport chain
The electron transport chain is initiated by the
reaction of an organic metabolite (intermediate in metabolic reactions) with
the coenzyme NAD+ (nicotinamide adenine dinucleotide is a coenzyme
containing the B-vitamin, nicotinamide). This is an oxidation reaction where 2
hydrogen atoms (or 2 hydrogen ions and 2 electrons) are removed from the
organic metabolite. (The organic metabolites are usually from the citric acid
cycle and the oxidation of fatty acids—details in following pages). The
reaction can be represented simply where M = any metabolite.

Complex
I - NADH dehydrogenase,
also called NADH coenzyme Q reductaselocated in the inner mitochondrial
membrane and also contains non heme iron atoms. These dehydrogenase enzyme does
not react with oxygen instead an electron carrier is interposed between the
metabolite and next member in the chain. These enzymes consist of a protein
part and a non protein part which is a coenzyme. The co enzyme NAD+ or NADP+
are utilized as the prime carriers of hydrogen.
Complex
II - Coenzyme Q (Q for Quinone)
or cytochrome c reductase is a Ubiquinone.It is in the inner membrane in the
free form or protein bound form. Coenzyme Q occupies the position between
metalloflavoproteins and cytochrome in the chain. At the point of coenzyme the
H+ ion dissociate and go into solution, leaving the electrons to the
cytochromes .
Complex
III -Cytochrome c
oxidase. Cytochromes are very similar to the structure ofmyoglobin or
hemoglobin. The significant feature is the heme structure containing the iron
(Fe) ions, initially in the +3 state and changed to the +2 state by the
addition of an electron. Cytochrome molecules accept only the electron from
each hydrogen, not the entire atom. The several types of cytochromes hold
electrons at slightly different energy levels. Electrons are passed along from
one cytochrome to the next in the chain, losing energy as they go. Finally, the
last cytochrome in the chain, cytochrome a3, passes two electrons to
molecular oxygen. These cytochromes are proteins that carry a prosthetic group
that has an embedded metal atom. The protein ‘steals’ the ability of the metal
atom to accept and release electrons.
Complex
IV - ATP synthase, also
known as the F0F1particle has two componentsF0
and F1 (F - indicates the factor). F1 protruding into
matrix from the inner membrane and F0 embedded and extend across the
inner membrane. The protruding F1 is essential for the energy
coupling to ATP molecule. Careful removal of this component (experimentally)
leads to impairment in ATP production though the intact respiratory chain is
present.
3. Reactions of electron transport
The electron acceptors in the electron transport
chain include FMN, ubiquinone (coQ), and a group of closely related proteins
called cytochromes. The figure 8.4 shows arrangement of the protein
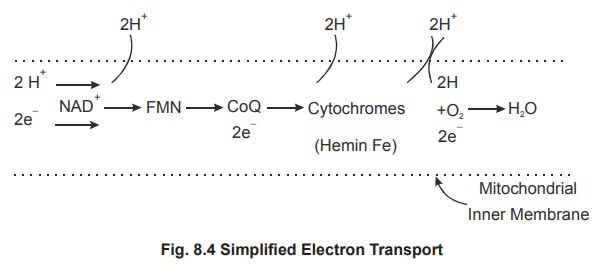
Oxidative Phosphorylation in Electron Transport
Chain consist of the electron donors
NADH+H+
FADH2
The coupled oxidation/reduction reaction is
NADH+H+ +1/2O2 ---------------> H2O +
NAD+
FADH2 + 1/2O2 -----------------> H2O + FAD
This coupled reactions yield free energy NADH+H+
yields 52 Kcal/mole as the electrons from NADH+H+ transfer to oxygen consist of
three pumps yield 3 ATP molecule at 3 sites FADH2 yields 36
Kcal/mole as the electron from FADH2 transfer to oxygen there are
two pumps yield 2 ATP molecule at 2 sites. The position at which the energy
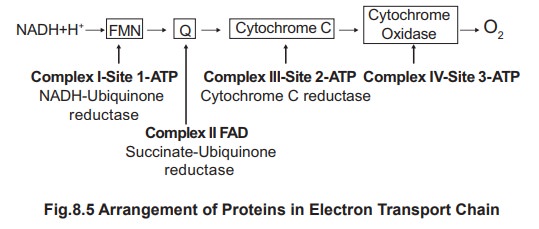
capture occurring as ATP are given in figure 8.5.
Spontaneous flow of electrons through each of
the respiratory chain complexes I, III, & IV is coupled to ejection of H+ from the mitochondrial
matrix to inner membrane space. The ejection of proton gradient is done through
inner membrane protein, ATPase that uses
released energy to drive the synthesis of ATP from ADP. The terminal
acceptor of electrons is molecular oxygen and it is reduced to water. However
not all the energy released are captured as high energy phosphate bond and
liberated as heat. In warm blooded animals this heat is used for the
maintenance of body temperature. The important respiratory control of electron
transport chainis the availability of ADP, the substrate for the ATP
Synthase.
4. Inhibitors of electron transport chain:
The use of inhibitors gives much information
about the electron transport chain. They are classified as (a) inhibitors of respiratory chain, (b) inhibitors ofoxidative
phosphorylation, and (c) uncouplers
of phosphorylation.
a. Inhibitors
that arrest respiration are
barbiturates like amobarbital, antibioticlike piericidin A , antimycin A and
fish poison retinone. The carbon
monoxideand cyanide inhibit cytochrome oxidase so that it cannot transport
electronsto oxygen. This blocks the further passage of electrons through the
chain, halting ATP production and life.
b. Inhibitors
of oxidative phosphorylation are
oligomycin and atrctyloside.
c. Uncouplers
dissolve in the
membrane, and function as carriers for
H+ or it can be an ionophores. Uncouplers block oxidative phosphorylation
by dissipatingthe H+ electro chemical gradient by an un coupling the essential
linkage between electron transport and ATP synthesis. Un couplers are 2,4
dinitro phenol, dinitrocresol, pentacholophenol.
Ionophores
(ion carriers) are
lipid soluble substance capable of carrying specificions through the membrane.
They slightly differ in their action from the uncouplers as they also transport
cation other than H+ through the membrane. Valiomycin forms a lipid complex
through which the K+ ion readily pass through. The ionophore gramicidin induces
penetration to H+, K+ or Na+ and uncouples the oxidative phosphorylation.
Related Topics