Chapter: Modern Pharmacology with Clinical Applications: Herbal Medicine
Herbal Medicine: Regulatory Issues
REGULATORY
ISSUES
The explosion in popularity of herbs dates to the Dietary Supplement Health and Education Act of 1994 (DSHEA), in which the FDA recognized herbal prepa-rations as dietary supplements outside of its direct reg-ulatory control. The act was a compromise between the FDA and manufacturing lobbies brought about in large measure by increased public demand for herbal prod-ucts. Instead of FDA regulation, these products now fall under the far less stringent Current Good Manu-facturing Practice in Manufacturing, Packaging or Holding Human Food regulations. Unlike prescription pharmaceuticals, which must be proved safe and effec-tive before being marketed, supplements do not have to be either safe or effective as long as they avoid thera-peutic claims on the label. Neither are they policed in regard to delivering accurate doses, although some consumer-oriented organizations, such as Consumer Lab (www.ConsumerLab.com), are starting to hold manufacturers more accountable through random test-ing and reporting of their results. Supplements are per-mitted to have “structure–function” statements on their label stating only the product’s supposed physiological function. For instance, an Echinacea product label might read “supports immune function” but may not claim to prevent or abort the common cold.
The FDA recommended in 1999 an improved dietary supplement labeling system with a
supplement facts panel that is be-ing gradually adopted by the industry. Future
legislation may further tighten the quite lax U. S. regulatory envi-ronment.
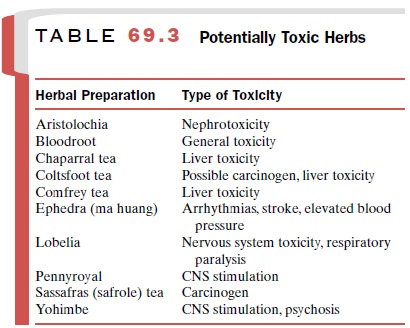
Unsafe herbs (Table 69.3) also will be increas-ingly restricted.
The European system differs
from that of the United States in that the manufacture of the more
phar-macologically active herbs is for the most part regulated and is much more
widely accepted by the medical com-munity. Many health care providers routinely
prescribe herbal treatments either alongside or in place of more conventional
medications. The German government’s Commission
E publishes a set of herbal monographs
that not only officially sets the standard for that country but has also
become a widely respected clinical refer-ence throughout the world.
Related Topics