Chapter: Modern Medical Toxicology: Hydrocarbons and Pesticides: Hydrocarbon
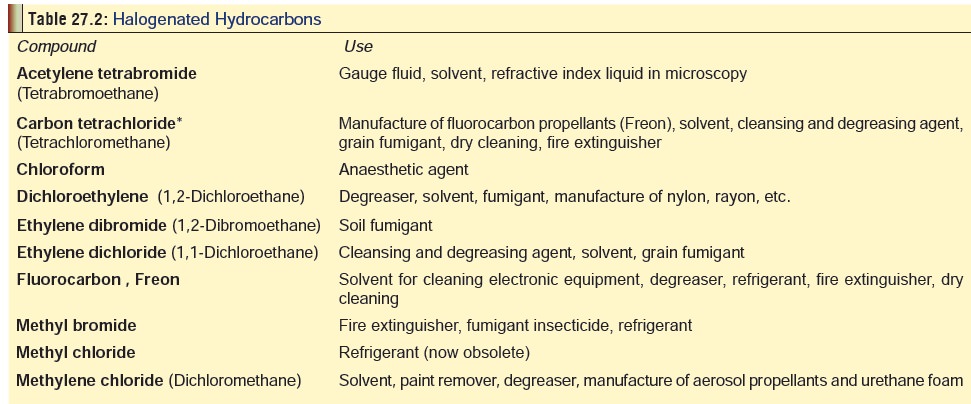
Halogenated Hydrocarbons
HALOGENATED HYDROCARBONS
Examples
Listed
in Table 27.2.

Physical Appearance
Most halogenated hydrocarbons are
clear, colourless, non-inflammable liquids with sweetish, chloroform-like
odour. Many of them also exist as gases. For instance, methyl bromide is a
toxic inhalant, and an intense vesicant, with dermal expo-sures resulting in
burns. It is a colourless, transparent, volatile liquid or gas with a burning
taste. It is nearly odourless, though chloropicrin is typically added to
commercial forms of methyl bromide to give it an intense odour.
Toxicokinetics
·
The usual route of exposure is
either inhalation or ingestion. Many halogenated hydrocarbons can be absorbed
through skin, albeit slowly.
·
After absorption they are
distributed mainly in the blood, brain, and adipose tissue.
· Metabolism occurs in the liver by cytochrome P450 oxida-tion. There is partial glutathione conjugation.
![]()
Mode of Action
■■ Most of these agents
are powerful hepatorenal toxins, producing centrilobular liver necrosis and
renal tubular degeneration.
■■ In the case of
carbon tetrachloride, the hepatic mixed-function oxidase system metabolises it
to the trichloro-methyl radical (CCl3.). This initiates lipid peroxidation,
protein-lipid cross links, and trichloromethyl adducts with DNA, protein and
lipid. The trichloromethyl radical may poison the cytochrome P 450. It may be
released from the cytochrome P 450 or may be converted to chloroform via a
one-electron reduction and abstraction of a proton. Further reduction may
release hydrochloric acid and carbon monoxide. The trichloromethyl radical may
alternatively react with oxygen to form a trichloromethyl peroxy free radical,
which may react to form phosgene. This may play a significant role in mediation
of carbon tetrachloride hepatotoxicity.
■■ Recent
studies have focused on intracellular calcium homoeostasis. The metabolism of
carbon tetrachloride disrupts the hepatocyte ATP dependant Ca++ pump. This
results in a rise of intracellular cytoplasmic Ca++. The latter may be a toxic
second messenger that activates mechanisms which destroy cellular membranes
resulting in cell death.
■■ Methyl bromide, and
possibly some other hydrocarbons, behave as alkylating agents and sulfhydryl
enzyme inhibitors in mammalian tissues. It has been speculated that hexokinase
and pyruvate oxidase may be especially susceptible to inactivation by
methylation of SH-groups in the CNS. The similarity of neuropathological
manifesta-tions of methyl bromide toxicity to those seen in thiamine deficiency
may be related to effects of methyl bromide interference with metabolism of
pyruvate, where thiamine acts as a co-factor.
Clinical Features
Acute Poisoning:
·
Vomiting, diarrhoea, abdominal pain, headache, leth-argy,
vertigo and stupor.
· Headache, fatigue, confusion, altered mental status, delirium, amnesia, incoherent speech, ataxia, inten-tion tremor, and positive Rhomberg sign may occur. Behavioural disturbances resembling psychosis may be noted as an early manifestation of methyl bromide toxicity.
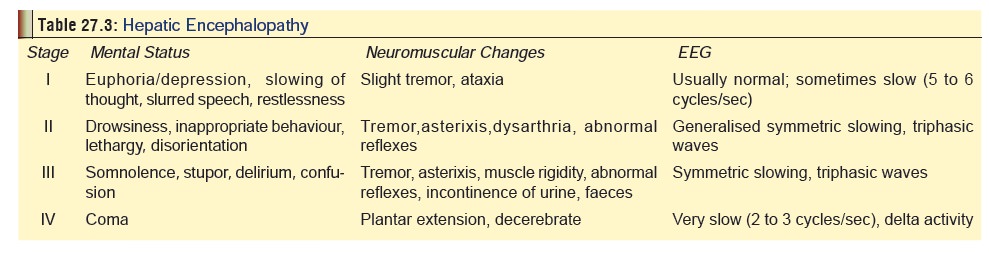
·
Liver damage results in hepatitis, jaundice, and hepatic
encephalopathy (Table 27.3).
·
Renal involvement is manifested by oliguria or anuria,
haematuria and renal failure.
·
Additional features include acidosis, hypertension,
convulsions and respiratory failure. Hypotension, ventricular arrhythmias,
depressed cardiac muscles, fatty degeneration, and a slowed or irregular pulse
may occur.
·
If alcohol has been consumed along with a halogenated
hydrocarbon, particularly carbon tetrachloride, there is rapid onset and
progression of symptoms.
·
Methyl bromide intoxication is characterised by myoclonic
convulsions and permanent brain damage. Signs and symptoms may include blurred
or double vision, nystagmus, hypotension, cough, tachypnoea, cyanosis,
lethargy, profound weakness, dizziness, slur-ring of speech, hyperreflexia,
albuminuria, haematuria, oliguria, anuria, and impaired liver function.
·
Dermal exposure (especially by methyl bromide) may result in
second degree burns. Methyl bromide is an intense vesicant with the capacity to
penetrate protective clothing. Skin blisters are produced, but are rarely deep
enough to destroy entire skin layer. Spillage of liquid fumigant on the skin is
likely to result in injury ranging from erythema to vesiculation. The
inflammation and blistering can be delayed for 15 to 20 hours. Healing is
gradual, often taking several weeks. Skin contact with many halogenated
hydrocarbons, especially carbon tetrachloride can lead to dermatitis through
defatting action. Erythema, hyperaemia, wheals, and vesicula-tions may be seen.
Gastrointestinal effects (abdominal pain, nausea, vomiting, diarrhoea) and renal
or hepatic damage can occur even from dermal exposure.
Chronic Poisoning:
·
Trichloroethylene (together with
ethanol) when used as a degreaser results in intermittent skin contact
producing flushing (Degreaser’s flush)
due to vasodilation of super-ficial skin vessels.
·
Chronic exposure to halogenated
hydrocarbon solvents can cause Painter’s
syndrome: headache, fatigue, memory lapses, irritability, depression, and
intolerance to alcohol.
·
Occurrence of a protracted
extrapyramidal syndrome following low-level methyl bromide exposure has been
documented in several cases. Depression, slow mentation, poor memory, neurosis,
muscle paralysis, and ataxia may be long-term or permanent disabilities
associated with methyl bromide poisoning. Other long-term effects include
myoclonus, difficult speech, cognitive impairment, muscular atrophy, peripheral
neuropathy and seizure disorders.
·
Chronic exposure to carbon
tetrachloride has been possibly associated with myasthenic reaction, a defect
in neuromuscular transmission.
·
There are reports suggesting that
some halogenated hydrocarbons are carcinogenic and may cause renal cancer
(especially carbon tetrachloride, tetrachloro-ethylene, and trichloromethane).
Effects of chronic exposure to carbon tetrachloride include liver cancer in
persons with acute poisoning, which might occur with prior chronic exposure,
even in the absence of cirrhosis, and a possible association with brain
tumours, lymphatic leukaemias and lymphosarcomas.
Usual Fatal Dose
About
4 to 5 ml for most halogenated hydrocabons; 20 to 25 ml for a few others. With
reference to methyl bromide, airborne concentrations as low as 100 ppm have
been reported to be harmful, while concentrations of 8,000 to 60,000 ppm may be
fatal.
Diagnosis
·
Characteristic odour in the breath.
·
Positive Fehling’s test (for sugar in the urine).
·
Isonitrile Test: 10 ml of distillate or a small
amount of thesuspected liquid in 10 ml of water is placed in a test tube.
·
To this, 1 ml of purified aniline and 2 ml of 20% sodium
hydroxide are added and gently heated. A positive result is indicated by the
development of a foul skunk-like odour due to formation of phenyl isonitrile.
·
Gas chromatography can be used to quantitate halogenated
hydrocarbons in biological samples.
·
Carbon tetrachloride blood levels in acutely poisoned
patients ranged from 0.1 to 31.5 mg/L. 2 to 5 mg/dL are generally considered
toxic blood levels.
·
Serum inorganic bromide levels may be useful in confirming
exposure to methyl bromide and may correlate with the clinical severity of poisoning.
Values in excess of 5 mg/100 ml bromide are generally toxic. However, this is
not always the case.
·
Hepatorenal toxicity is indicated by elevated serum hepatic
aminotransferase, bilirubin, alkaline phosphatase, and creatinine.
·
Individual serum bile acids appear to be very sensitive
indi-cators of liver damage and may be used as early indicators of carbon
tetrachloride-induced liver injury as measured by high performance liquid
chromatography. This appears to be much more sensitive than measuring liver
enzyme or bilirubin levels.
·
A chest radiograph should be considered in patients with
respiratory symptoms. Carbon tetrachloride is radiopaque, and some ingestions
may be able to be confirmed with an abdominal radiograph.![]()
Treatment
·
Decontamination—dermal exposure should be treated by
stripping the patient nd washing copiously with soap and water. Eye involvement
must be treated by irrigation for at least 15 to 20 minutes. Consider
administration of activated charcoal after a potentially toxic ingestion. Gastric
lavage can also be done cautiously in potentially lethal ingestions.
·
Administer oxygen if there is evidence of altered mental
status or respiratory failure.
·
Watch out for cardiac arrhythmias, aspiration pneumo-nitis,
and hepatorenal failure.
·
Carbon tetrachloride-induced liver cirrhosis results in bile
acids not being detoxified in the enterohepatic circulation.
·
In rat studies administration of cholestyramine, which has a
strong affinity for bile acids in the intestine, prevents their enteral
resorption and decreases the induction of cirrhosis.
·
N-acetylcysteine given within 8 to 10 hours after expo-sure
has been reported to prevent hepatic damage from acute poisoning by CCl4
in humans. It is probably most effective if given within 16 hours following ingestion
of carbon tetrachloride. Further studies are needed before this therapy can be
routinely recommended. Estimated dose of NAC: Loading dose of 140 mg/kg orally
as a 5% solution in cola followed by a maintenance dose of 70 mg/kg orally
every 4 hours for 17 doses. Alternatively, the Prescott protocol can be followed: gastric lavage followed by
intravenous infusion of N-acetylcysteine at 150 mg/kg over 15 minutes, then 50
mg/kg over 4 hours, followed by 100 mg/kg over 16 hours.
·
Intravenous administration of N-acetylcysteine has been
suggested as a treatment for methyl bromide poisoning also, possibly based on
the hypothesis that methyl bromide preferentially reacts with dermal SH-groups.
N-acetylcysteine would serve as a source of SH-groups to react with unbound
methyl bromide. However, this treatment cannot be recommended until further
studies are done to confirm efficacy.
· Treat renal failure with dialysis and hepatic failure with fresh frozen plasma, vitamin K, low-protein diet, neomycin and lactulose.
·
Hyperbaric oxygen significantly improved survival and
decreased the degree of SGPT elevation in rats poisoned with carbon
tetrachloride. A review of subsequent litera-ture suggests that hyperbaric
oxygen treatment is appro-priate treatment for carbon tetrachloride
intoxication.
·
Haemodialysis is generally not effective, though an
anecdotal report suggests it may be useful in methyl bromide poisoning.
Haemodialysis or haemoperfusion may be necessary to support patients in renal
or hepatic failure, respectively.
·
Treatment of dermal burns (methyl bromide):
·
![]() After initial flushing with large
volumes of water to remove any residual chemical material, clean wounds with a
mild disinfectant soap and water.
After initial flushing with large
volumes of water to remove any residual chemical material, clean wounds with a
mild disinfectant soap and water.
·
Loose, nonviable tissue should be removed by gentle
cleansing with surgical soap or formal skin debride-ment. Intravenous analgesia
may be required.
·
Removal and debridement of closed blisters is
contro-versial. Current consensus is that intact blisters prevent pain and
dehydration, promote healing, and allow motion; therefore, blisters should be
left intact until they rupture spontaneously or healing is well underway,
unless they are extremely large or inhibit motion.
·
Prophylactic topical antibiotic therapy with silver
sulfadiazine is recommended for all burns except superficial partial thickness
(first-degree) burns. Systemic antibiotics are generally not indicated unless
infection is present or the burn involves the hands, feet, or perineum.
·
Depending on the site and area, the burn may be treated open
(face, ears, or perineum) or covered with sterile nonstick porous gauze. The
gauze dressing should be fluffy and thick enough to absorb all drainage.
Alternatively, a petrolatum fine-mesh gauze dressing may be used alone on
partial-thickness burns.
·
Analgesics such as paracetamol with codeine may be used for
pain relief if needed.
·
Tetanus toxoid 0.5 ml intramuscularly (or other indi-cated
tetanus prophylaxis) should be administered if required.
Autopsy Features
·
Characteristic odour.
·
Petechiae in the brain, airways, and lungs.
·
Pulmonary oedema.
·
Fatty degeneration, cardiomegaly
·
Renal and hepatic necrosis. Large foci of centrilobular
necrosis of the liver with normal portal vasculature was reported at autopsy of
a 36-year-old female following a fatal methyl bromide exposure.
Forensic Issues
Most cases are accidental in nature
arising out of occupa-tional exposure. There have been cases of suicidal
ingestion involving one or other of these compounds.
Related Topics