Heat | Term 2 Unit 1 | 6th Science - Flow of Heat | 6th Science : Term 2 Unit 1 : Heat
Chapter: 6th Science : Term 2 Unit 1 : Heat
Flow of Heat
Flow
of Heat
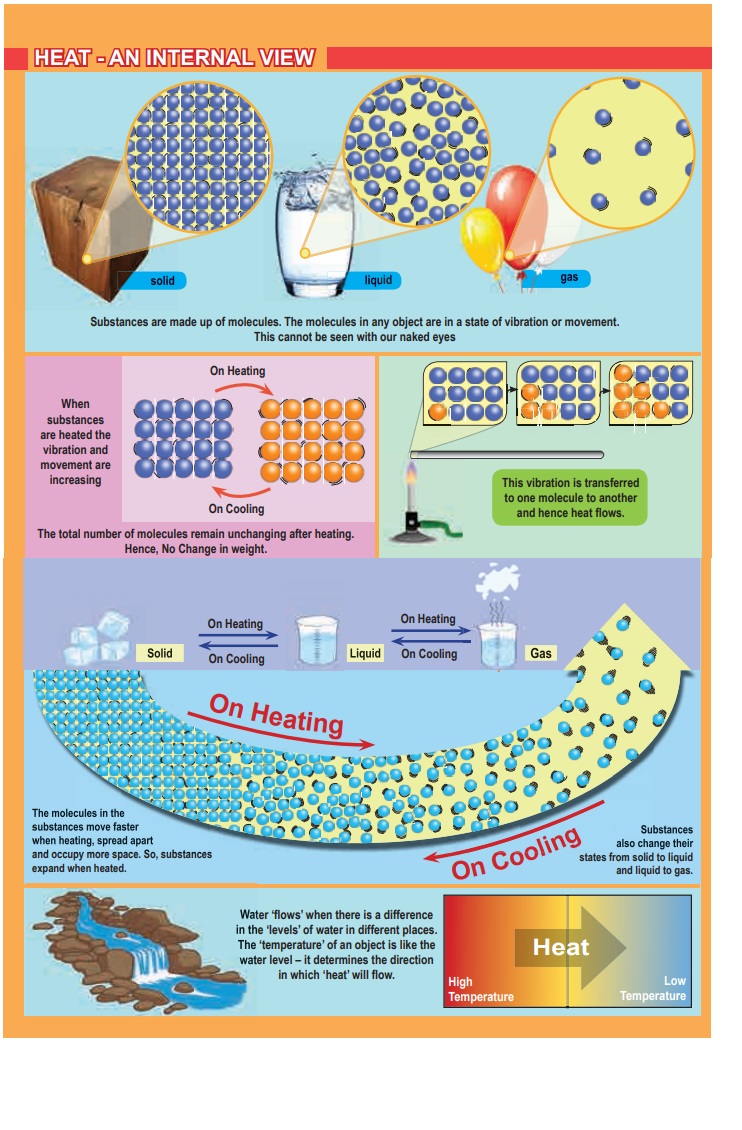
An analogy between temperature and water level:
Water ‘flows’ when there is a difference in the ‘levels’ of water
in different places. It does not matter if there is more water in one place or
another. Water from a puddle can flow into a reservoir or the other way around.
The ‘temperature’ of an object is like the water level – it determines the direction in which ‘heat’
will flow. Heat energy flows from higher temperature to lower temperature.
Thermal
contact and Thermal equilibrium
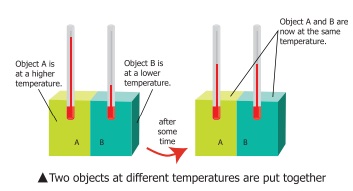
Consider two bodies A and B. Let the temperature
of A be higher than that of B. On bringing bodies A and B in contact , heat
will flow from hot body A to the cold body B. Heat will continue to flow till
both the bodies attain the same temperature.
The
temperature determines the direction of flow of heat.
1.
You are holding a hot cup of coffee. Would the Heat energy transfer from

a.
Your body to the coffee, or
b. The coffee to your body?
The heat energy gets transferred from the coffee to my
body.
2.
You are standing outside on a summer day. It is 40ºC outside (note that normal
body temperature is 37ºC). Would the Heat energy transfer from.
a.
Your body to the air particles, or
b.
The air particles to your body?

Heat energy flows from air particles to my body.
3.
You are standing outside on a winter day. It is 23ºC outside. Would the heat
energy transfer from:
a.
Your body to the air particles, or
b.
The air particles to your body?

The heat energy transfer is from my body to the air particles.
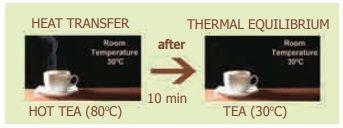
Two objects are said to be in thermal contact
if they can exchange heat energy. Thermal equilibrium exists
when two objects in thermal contact no longer affect each other's temperature.
For example, if a pot of milk from the
refrigerator is set on the kitchen table, the two objects are in thermal
contact. After certain period, their temperatures are the same, and they are
said to be in thermal equilibrium.
Related Topics