Chapter: Obstetrics and Gynecology: Infertility
Evaluation of Infertility
EVALUATION OF INFERTILITY
The most common causes of male and female infertility are investigated during the initial evaluation of infertility. It is important to recognize that more than one factor may be involved in a couple’s infertility (Table 38.1). As with any medical condition, a careful history and evaluation should reveal factors that may be involved in a couple’s infertility, such as medical disorders, medications, prior surgeries, pelvic infections or pelvic pain, sexual dysfunc-tion, and environmental and lifestyle factors (e.g., diet, exercise, tobacco use, drug use).
The timing of the initial evaluation depends primar-ily on the age of the female partner and a couple’s risk fac-tors for infertility. Because there is a decline in fecundity withadvancing maternal age, women over age 35 may benefit from a preliminary evaluation before 12 months of attempted conception. The initial assessment and treatment of infertility is com-monly provided by an obstetrician-gynecologist. More specialized evaluation and treatment may be performed by a reproductive endocrinologist.
Ovulation
A history of regular, predictable
menses strongly suggests ovulatory cycles. Furthermore, many women experience
characteristic symptoms associated with ovulation and the production of
progesterone: unilateral pelvic discomfort (mittelschmerz), fullness and
tenderness of the breasts, decreased vaginal secretions, abdominal bloating,
slight increase in body weight, and occasional episodes of depres-sion. These changes
rarely occur in anovulatory women. Therefore, a history of regular menses with
associated cyclic changes may be considered presumptive evidence of ovulation.
Secretion
of progesterone by the corpus luteum dominates the luteal phase of the menstrual
cycle, and persists if conception occurs.
Progesterone acts on the endocervix to convert the thin, clear endocervical mucus into a sticky mucoid material. Progesterone also changes the brain’s thermoregulatory center setpoint, resulting in a basal body temperature rise of approximately 0.6°F. In the absence of pregnancy, invo-lution of the corpus luteum is associated with an abrupt decrease in progesterone production, normalization of the basal body temperature, and the commencement of menstruation.
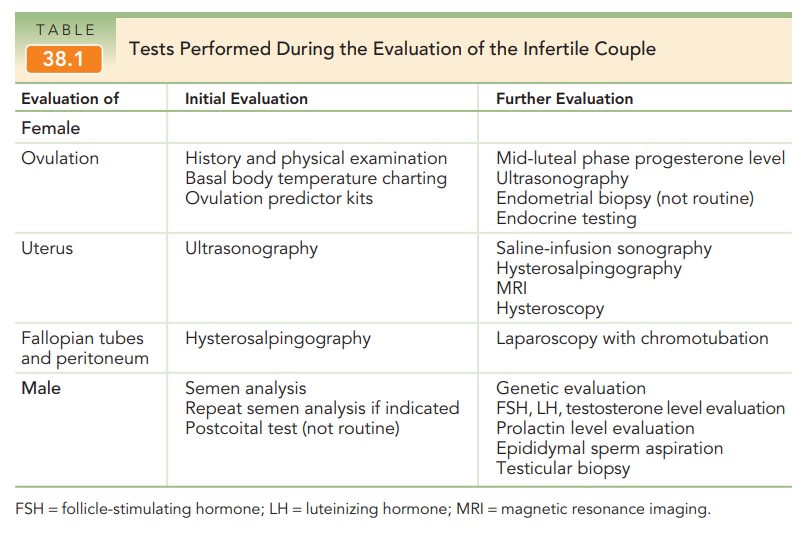
Two tests provide indirect evidence of ovulation and can help predict the timing of ovulation. Basal body tempera-ture measurement reveals a characteristic biphasic tem-perature curve during most ovulatory cycles (Fig. 38.3). Special thermometers are available for this use. Upon awak-ening in the morning, the temperature must be obtained immediately before any physical activity.
The temperature drops at the time of menses, and
then rises 2 days after the peak of the luteinizing hormone (LH) surge,
coinciding with a rise in peripheral levels of progesterone. Oocyte release
occurs 1 day before the first temperature elevation, and the temperature
remains elevated for up to 14 days. This test for ovulation is readily
available, although cum-bersome to use; it can retrospectively identify
ovulation and the optimal time for intercourse, but can be difficult to
interpret. Urine LH kits are also
used to prospec-tively assess the presence and timing of ovulation based on
increased excretion of LH in the urine. Ovulation occurs approximately 24 hours
after urinary evidence of the LH surge. However, due to the pulsatile nature of
LH release, an LH surge can be missed if the test is only performed once daily.
Other diagnostic tests assess
ovulation using serumprogesterone levels
and the endometrial response toprogesterone.
A mid-luteal phase serum progesterone level can be used to retrospectively
assess ovulation. A value above 3 ng/mL implies ovulation; however, values
between 6 to 25 ng/mL may occur in a normal ovulatory cycle. Due to the
pulsatile nature of hormone secretion, a single low progesterone assessment
should be repeated. Another diagnostic procedure is the luteal phase endome-trial biopsy. The identification of secretory
endometriumconsistent with the day of the menstrual cycle confirms the presence
of progesterone; hence ovulation is implied. However, this procedure is
invasive, and histologic assess-ment of the endometrium does not reliably
differentiate infertile and fertile women. Therefore, the endometrial biopsy is
not routinely performed to assess ovulation or the endometrium.
If oligo-ovulation (sporadic and unpredictable ovula-tion) or anovulation (absence of ovulation) is
established, usually based on a menstrual cycle history of irregular cycles,
further testing is indicated to determine the underly-ing cause. A common cause
of ovulatory dysfunction in reproductive-age women is polycystic ovary syndrome (PCOS); other causes include thyroid
disorders and hyper-prolactinemia. Women with PCOS often present with
oligomenorrhea and signs of hyperandrogenism such as hir-sutism, acne, and
weight gain. Furthermore, some infertile women present with amenorrhea, and this usually signifies anovu-lation.
Important causes of amenorrhea include pregnancy (a pregnancy test should
always be given), hypothalamic dysfunction (usually stress-related), ovarian
failure, or obstruction of the reproductive tract. Depending on the individual
case, laboratory testing for ovulatory dysfunction may include assessment of
serum levels of human chori-onic gonadotropin (hCG), thyroid-stimulating
hormone (TSH), prolactin, total testosterone, dehydroepiandros-terone sulfate
(DHEA-S), follicle-stimulating hormone (FSH), LH, and estradiol.
Treatment
of the etiology of ovulatory dysfunction may lead to resumption of ovulation
and improved fertility.
Anatomic Factors
The pelvic anatomy should be
assessed as a part of the infer-tility evaluation. Abnormalities of the uterus,
fallopian tubes, and peritoneum can all play a role in infertility.
UTERUS
Uterine abnormalities are
commonly not sufficient to cause infertility; these disorders are usually
associated with preg-nancy loss. However,
assessment of the uterus is particularlyimportant if there is a history that
causes concern, such as abnor-mal bleeding, pregnancy loss, preterm delivery,
or previous uterine surgery. Potential uterine abnormalities include
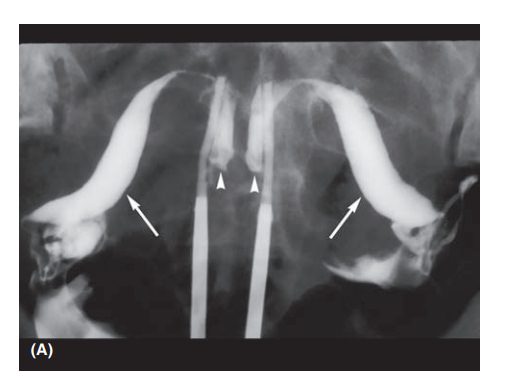
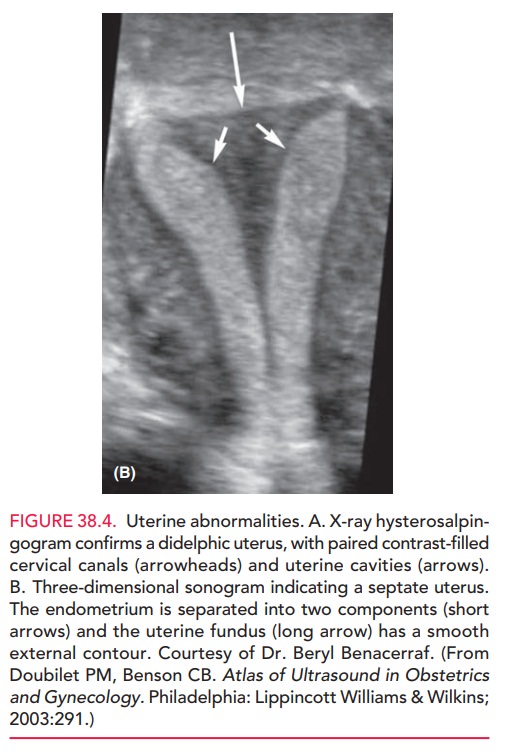
leiomyomas,endometrial polyps, intrauterine adhesions, or congenital anomalies
(such as a septate, bicornuate, unicornuate, or didelphyic uterus) (Fig. 38.4).
Assessment of the uterus and endometrial cavity can be accomplished with
several imag-ing techniques; sometimes, a combination of modalities is
necessary to best assess pelvic anatomy (Box 38.1).
FALLOPIAN TUBES AND PERITONEUM
The
fallopian tubes are dynamic structures that are essential for ovum, sperm, and
embryo transport and fertilization.
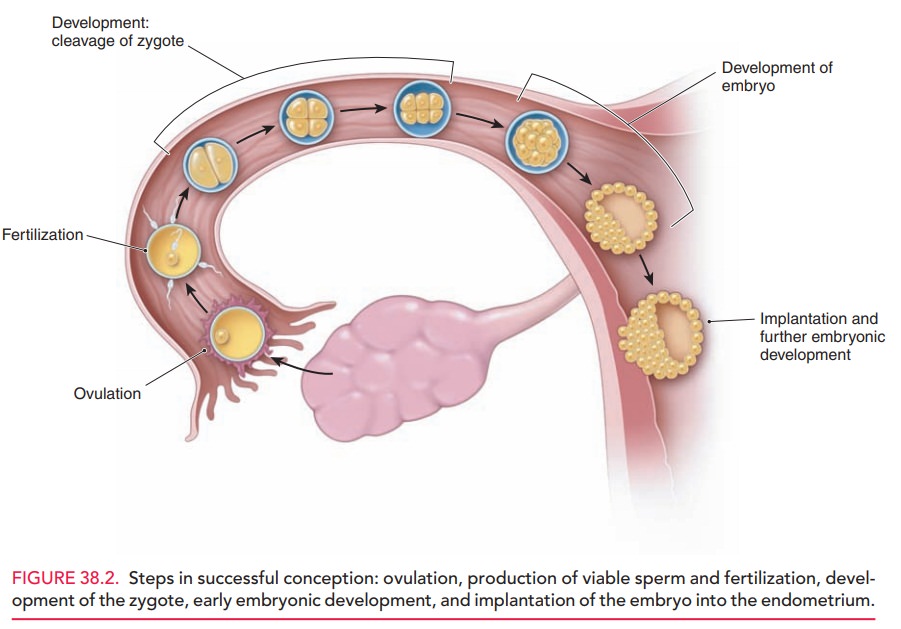
At ovulation, the fimbriated end of the fallopian tube picks up the oocyte from the site of ovulation or from the pelvic cul-de-sac. The oocyte is transported to the ampullary portion of the fallopian tube where fertilization occurs (see Fig. 38.2). Subsequently, a zygote and then an embryo are formed. At 5 days following fertilization, the embryo enters the endometrial cavity, where implantation into the secretory endometrium occurs, followed by further embryo growth and development.
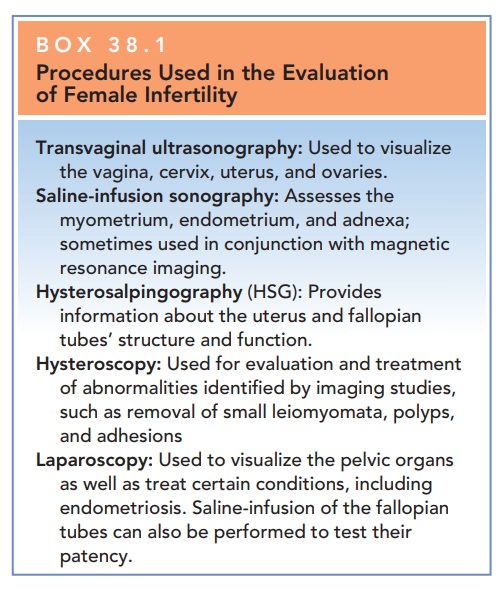
Box 38.1
Procedures Used in the Evaluation of Female Infertility
Transvaginal
ultrasonography: Used to visualize the vagina, cervix, uterus, and ovaries.
Saline-infusion
sonography: Assesses the myometrium, endometrium, and adnexa; sometimes used in
conjunction with magnetic resonance imaging.
Hysterosalpingography
(HSG): Provides information about the uterus and fallopian tubes’ structure and
function.
Hysteroscopy:
Used for evaluation and treatment of abnormalities identified by imaging
studies, such as removal of small leiomyomata, polyps, and adhesions
Laparoscopy: Used to visualize the pelvic organs as well as treat certain conditions, including endometriosis. Saline-infusion of the fallopian tubes can also be performed to test their patency.
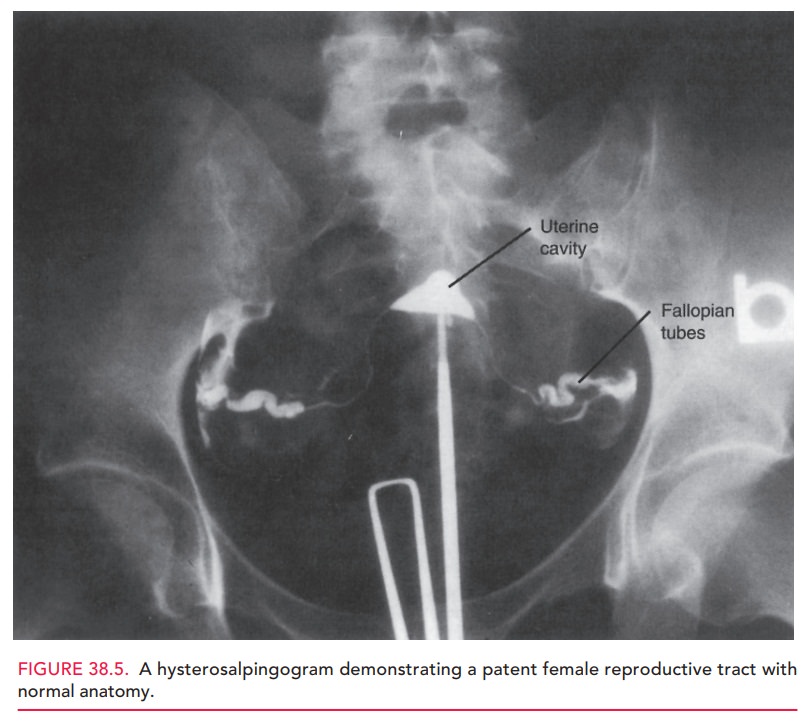
The fallopian tubes and pelvis can be evaluated with hysterosalpingography (HSG) or laparoscopy. There are several important
characteristics of a normal HSG (Fig. 38.5). The uterine cavity should be
smooth and sym-metrical; indentations or irregularities of the cavity suggest
the presence of leiomyomas, endometrial polyps, or intra-uterine adhesions. The
proximal two-thirds of the fallo-pian tube should be thin, approximating the
diameter of a pencil lead. The distal one-third comprises the ampulla, and
should appear dilated in comparison to the proximal portion of the tube. Free
spill of dye from the fimbria into the pelvis is appreciated as the cul-de-sac
and other struc-tures such as bowel are outlined by the accumulating dye. Failure
to observe dispersion of dye through a fallopian tube or throughout the pelvis
suggests the possibility of pelvic adhesions that restrict normal fallopian
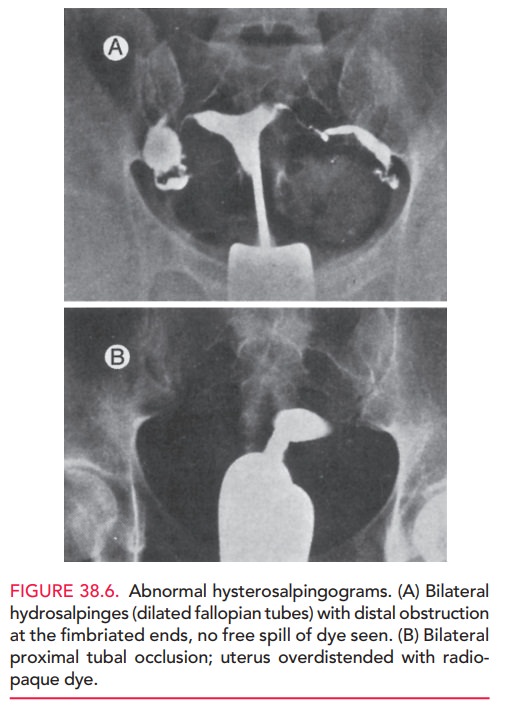
tube mobil-ity. Examples of abnormal hysterosalpingograms are shown in Figure
38.6.
Pelvic adhesions that affect the fallopian tubes or peri-toneum may occur because of pelvic infection (e.g., pelvic inflammatory disease, appendicitis), endometriosis, or abdominal or pelvic surgery. The sequelae of any of these processes or events can include fallopian tube scar-ring and obstruction. Pelvic infections are usually associated with sexually transmitted infections that cause acute sal-pingitis; commonly implicated organisms are Chlamydia trachomatis and Neisseria gonorrhea. Endometriosis occurs with higher frequency in infertile women compared to fertile women, and can cause scarring and distortion of the fallopian tubes and other pelvic organs.
The HSG detects approximately 70%
of anatomic abnormalities of the genital tract. When there are abnormal-ities,
further diagnostic evaluation and treatment can be per-formed with hysteroscopy
and laparoscopy. Hysteroscopy evaluates the endometrium and the architecture of
the uter-ine cavity. Laparoscopy assesses pelvic structures including the
uterus, ovaries, and fallopian tubes as well as the pelvic peritoneum. During
laparoscopy, chromotubation should
be performed: similar to the HSG, a catheter is placed in the uterus and
colored dye is injected into the uterus while tubal patency and function is
directly assessed by laparoscopy. Laparoscopy also allows the diagnosis and
treatment of any pelvic abnormalities, such as adhesions and endometriosis.
Related Topics