Chapter: Modern Pharmacology with Clinical Applications: Mechanisms of Drug Action
Equations Derived From Drug–Receptor Interactions
EQUATIONS
DERIVED FROM DRUG–RECEPTOR INTERACTIONS
It is important not to
confuse the term potency with affinity or the term intrinsic activity with
efficacy. The constants that
relate an agonist A and its receptor R to the response may be represented as
follows:
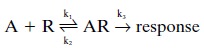
Affinity is k1/k2,
and efficacy is represented by k3. Thus, affinity and efficacy
represent kinetic constants that re-late the drug, the receptor, and the
response at the mo-lecular level. Affinity is the measure of the net molecu-lar
attraction between a drug (or neurotransmitter or hormone) and its receptor.
Efficacy is a measure of the efficiency of the drug–receptor complex in
initiating the signal transduction process. In contrast, potency and in-trinsic
activity are simple measurements, respectively, of the relative positions of
dose–response curves on their horizontal axes and of their relative maxima.
Affinity is one of the determinants
of potency; efficacy contributes both to
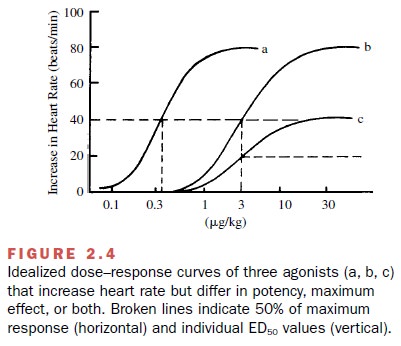
potency and to the maximum effect of the ago-nist. Figure 2.4 shows that drug c has less efficacy (and less intrinsic
activity) than either drug a or drug b. However, in contrast to intrinsic
activity, no numerical value of efficacy can be calculated from the data
pre-sented. Unfortunately, the terms potency
and efficacy are frequently used in a
loose and misleading manner.
The mathematical relationship
of response to effi-cacy and affinity is the following:
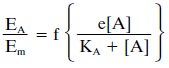
This equation states that the
ratio of the response (EA) to a given concentration of an agonist to
the maximum response (Em) of the test system, such as an isolated
strip of muscle, is a function (f) of efficacy (e) times the concentration of
the agonist ([A]) divided by the disso-ciation constant (KA) plus
the concentration of the ago-nist. KA is the reciprocal of the
affinity constant and, un-der equilibrium conditions,
KA = [R][A] / [RA]
[R] is the concentration of
free receptors and [RA] is the concentration of receptors bound to agonist. It
should be noted that by the use of combinations of agonists and antagonists,
dose–response curves, and mathematical relationships, it is possible to estimate
the dissociation constants of agonists and antagonists for a given receptor and
to estimate the relative efficacy of two agonists acting on the same receptor.
Related Topics