Chapter: Biochemistry: Enzyme Kinetics
Enzyme Inhibitor - Concepts
Enzyme Inhibitor - Concepts
The rates of enzyme catalysed reactions are
decreased by specific inhibitors. Inhibitors are compounds that combine with
enzymes and prevent enzyme and substrate from forming ES complex. The toxicity
of many compounds such as hydrogen cyanide and hydrogen sulphide results from
their action as enzyme inhibitors. Many drugs also act to inhibit specific
enzymes. Thus, knowledge of enzyme inhibitors is vital to understand drug
action and toxic agents.
Compounds which convert the enzymes into
inactive substances and then adversely affect the rate of enzyme catalysed
reactions are called as enzyme inhibitors. Such a process is known as enzyme
inhibition. Two broad classes of enzyme inhibitors are generally recognized.
They are reversible and irreversible inhibitors. This depends on whether the
inhibition can be reversed or not.
1. Reversible Enzyme Inhibition
A reversible enzyme inhibitor dissociates very
rapidly from its target enzyme because it becomes very loosely bound with the
enzyme. Three general types of reversible inhibition is observed: competitive,
noncompetitive and un-competitive, depending on the following factors.
·
Whether
the inhibition is over come or not by increasing the concentration of the
substrate.
·
Whether
the inhibitor binds with the active site or site other than the active site
(allosteric site).
·
Whether
the inhibitor binds with the free enzyme only or with the enzyme substrate
complex only or with either of the two.
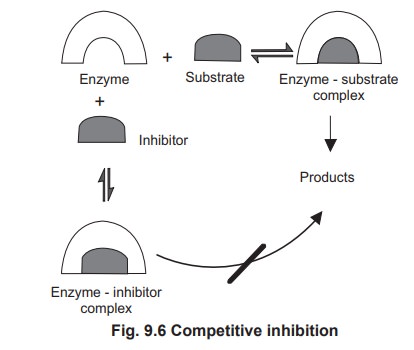
1.1 Competitive Inhibition
Competitive inhibitors can combine reversibly
with the active site of enzyme and compete with the substrate for binding with
the active site. If the site is occupied by the inhibitor it is unavailable for
the binding of the substrate (Fig 9.6).
The competitive inhibitor always resembles the structure of the substrate. In
some cases competitive inhibitors are exact structural analogues of the
substrates. The combination of a competitive inhibitor (I) with enzyme (E) can
be written in the same manner as combination with substrate, although the inhibitor
cannot be chemically transformed to products.
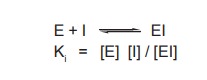
Ki is equal to the dissociation
constant for the enzyme – inhibitor complex EI.
The degree of inhibition depends upon the
relative concentration of the substrate and the inhibitor. It also depends on
the relative affinity of inhibitor towards enzyme active site. Thus, by
increasing the substrate concentration we can decrease the degree of inhibition
keeping inhibitor concentration at constant level.
The classic example is the inhibition of
succinate dehydrogenase by malonate and other dicarboxylic acids. Succinate
dehydrogenase is a member of the group of enzymes catalyzing the Krebs
tricarboxylic acid cycle.
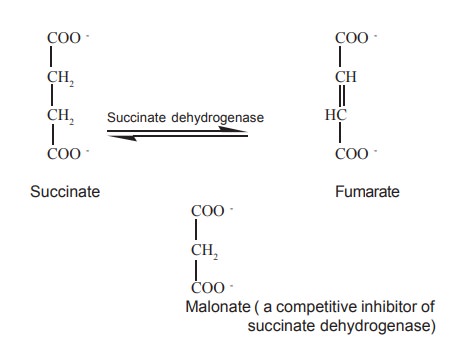
It catalyzes the removal of two hydrogen atoms
from the two methylene carbon atoms of succinate. Succinate dehydrogenase is
inhibited by malonate, which resembles succinate in having two ionized carboxyl
groups.
Many micro organisms like bacteria synthesize
the vitamin folic acid from para-aminobenzoic acid. Sulphanilamide and other
sulfa drugs are structural analogs of para-aminobenzoic acid. So, sulfa drugs
act as competitive inhibitor and occupy the active site of some bacterial
enzyme catalyzing this reaction. When this reaction is affected, it blocks the
folic acid biosynthesis which is essential for the growth of micro organisms,
ultimately results in the death of the micro organisms. Thus, many sulfa drugs
act as antibiotics
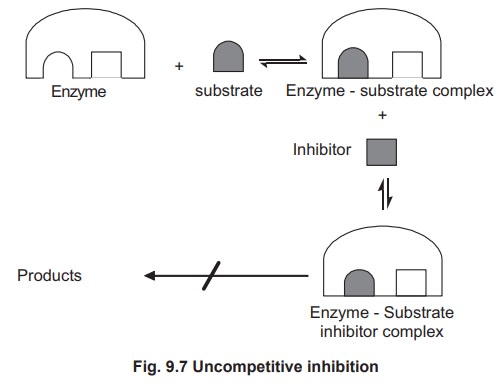
1.2. Un-competitive Inhibition
This type of inhibition occurs when an inhibitor
combines reversibly only with ES to form ESI which cannot yield the products.

Ki = dissociation constant of ESI
complex.
An un-competitive inhibitor also binds at an
allosteric site and the binding takes place only in enzyme substrate complexes
and not with the free enzyme molecule (Fig 9.7).
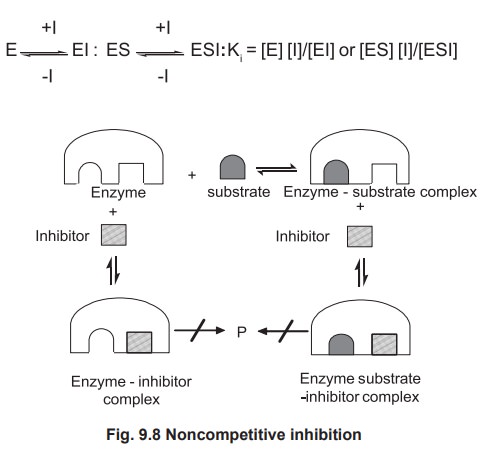
1.3. Non-competitive Inhibition
In this type of inhibition no competition occurs
between the substrate and the inhibitor and the inhibitor has no structural
resemblance with the substrate and it binds with the enzyme at a place other
than the active site. Since I and S may combine at different sites, formation
of both EI and ESI complexes takes place (Fig 9.8). The enzyme is inactivated
when inhibitor is bound, whether the substrate is present or not. Non
competitive inhibition in contrast to competitive inhibition cannot be overcome
by increasing substrate concentration. For example various heavy metal ions
such as Ag2+, Hg2+, Pb2+ inhibit the activity
of a variety of enzymes. Urease can be inactivated by any one of these metal
ions.
2. Irreversible Enzyme Inhibition
Irreversible inhibitors are those that combine
with or destroy a functional group on the enzyme that is essential for its
activity. The irreversible inhibitor dissociates very slowly from its target
enzyme because it becomes very tightly bound to its active site, thus
inactivating the enzyme molecule. The bonding between the inhibitor and the
enzyme may be covalent or noncovalent.
Examples of Irreversible Inhibition
·
Alkylating
agents such as iodoacetamide, irreversibly inhibit the catalytic activity of
some enzymes by modifying cysteine and other side chains.
·
Organo
phosphorous compounds such as diisopropyl phosphoflouridate are potential
irreversible inhibitors of enzymes that have active seryl residues at their
catalytic sites.