Chapter: Biochemistry: Enzyme Kinetics
Enzyme Action
Enzyme Action
The molecular events that accompany the
conversion of substrate into products constitute the mechanism of enzyme
action. Enzyme action on its substrate results either in the formation or
degradation of chemical bonds in the substrate molecules.
1. ES Complex Formation
According to Michaelis – Menton theory, the
enzyme E combines with the substrate S to form an intermediate enzyme substrate
complex ES. This complex then breaks down into product P and enzyme E is
regenerated. The enzyme can again combine with the fresh molecule of the
substrate in similar manner. The formation of enzyme substrate complex as an
intermediate during the reaction has been proved by spectroscopic studies. So,
a simple enzymatic reaction might be written as
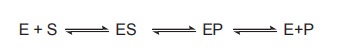
Where E, S and P represent enzyme, substrate and
product respectively. ES and EP are complexes of the enzyme with substrate and
product respectively. At the end of the reaction along with the required
products the enzyme is regenerated in its original form and can involve in
another round of catalysis. ES complex is a highly energised, transiently
existing complex which can be easily degraded to form the product.
In the formation of enzyme substrate complexes,
the substrate molecules attach at certain specific sites on the enzyme
molecules. These specific points on enzyme molecules where the substrate
molecules attach are known as active site or catalytic site.
Active sites on the enzymes are usually provided
by certain functional group of amino acids present in the enzyme protein. For
example, free hydroxyl group of serine, phenolic group of tyrosine, sulfhydryl
group of cysteine and imidazolyl group of histidine are some of the important
catalytic groups present in enzyme active sites.
2. Theories of Active Site
In 1894, Fischer proposed that the substrate fits
into the active site of the enzyme as a key fits into the lock (Fig 9.4). Because of this model, the
theory is known as lock and key theory of enzyme action.
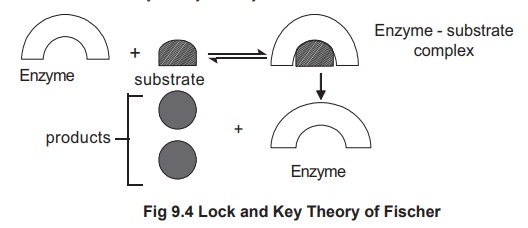
According to lock and key theory, there are
exact functional groups and structural features in the enzyme into which
substrate molecule must fit. The region of the enzyme that complexes with the
substrate is called active site or catalytic site. The theory cannot be applied
for all the enzymatic reactions because in some reactions the substrate
molecules and the active site are not structurally similar to fit in with each
other. Moreover, in certain cases the catalytic activity is observed even
though a fit is impossible.
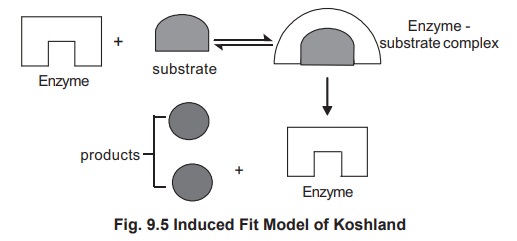
Later, lock and key theory was modified by
Koshland in 1963 in the form of ‘induced fit mechanism’. The essential feature
of this theory is the flexibility of the enzyme active site. In Fisher model,
the active site is presumed to be a rigid preshaped structure to fit the
substrate, while in the induced fit model the substrate induces the
conformational change in the enzyme (Fig
9.5), so that the substrate and active site come close to each other in
such a way that the substrate fits the active site in a more convenient manner.
The active site on the enzyme molecule exerts a
binding force on the substrate molecule by hydrophilic and hydrophobic
catalytic groups. Enzyme substrate complexes are formed by multiple bonding
i.e., covalent, electrostatic and hydrogen bonding with the substrate. The
functional group at the active site are arranged in a definite spatial manner
so that the ES complex formation is favourable.
Many enzymes require non proteinous group called
as coenzymes for their maximal activity. The enzymes requiring coenzymes for
their activity also possess sites for the attachment of co-enzymes. The
complexes formed in such cases are known as enzyme-substrate-coenzyme
complexes.
Certain enzymes require a metal ion, in addition
to coenzyme for their full activity. These metallic ions are called positive
modifiers of enzyme activity. Examples of such enzymes include alcohol
dehydrogenase, peroxidase, catalase and xanthine oxidase etc., which contain
sites for binding metal ions. The removal of metal from these enzymes often
results in partial or total loss of enzymatic activity. These enzymes are
otherwise called as metallo enzymes. The common metallic ions required for
enzymatic activity are K+, Cu+, Mg++, Ca++ etc.