Chapter: Essentials of Psychiatry: Childhood Disorders: Elimination Disorders and Childhood Anxiety Disorders
Enuresis
Enuresis
Definition
Functional enuresis is usually defined as the
intentional or involun-tary passage of urine into bed or clothes in the absence
of any iden-tified physical abnormality in children older than 4 years of age.
It is often associated with psychiatric disorder and enuretic children are
frequently referred to mental health services for treatment.
Course and Natural History
The acquisition of urinary continence at night is
the end stage of a fairly consistent developmental sequence. Bowel control
dur-ing sleep marks the beginning of this process and is followed by bowel
control during waking hours, bladder control during the day, and finally
night-time bladder control. Most children achieve this final stage by the age
of 36 months. With increasing age, the likelihood of spontaneous recovery from
enuresis decreases. The chronic nature of the condition is further shown in the
study by Rutter and colleagues (1973), in which only 1.5% of 5-year-old
bed-wetters became dry during the next 2 years.
Enuresis (Not Due to a Medical Condition)
This
disorder is characterized by the repeated voiding of urine into clothes or bed
at the age of 5 (chronicalogically or devel-opmentally) or older for a period
of at least twice weekly over three months. Enuresis reflects a significant
emotional distress or impairment in social, academic or other important ares of
functioning. Enuresis may be either diurnal (during waking
hours) or nocturnal (during
night-time sleep) or a combination of both.
Nocturnal enuresis is as common in boys as girls
until the age of 5 years, but by age 11 years, boys outnumber girls 2 : 1. Not
until the age of 8 years do boys achieve the same levels of night-time
continence that are seen in girls by the age of 5 years, probably due to slower
physiological maturation in boys. In addition, the increased incidence of
secondary enuresis (occurring after an initial 1-year period of acquired
continence) in boys further affects the sex ratio seen in later childhood.
Daytime enuresis occurs more commonly in girls and is associated with higher
rates of psychiatric disturbance.
Etiology and Pathophysiology
Possible biological factors include a structural
pathological con-dition or infection of the urinary tract (or both), low
functional bladder capacity, abnormal antidiuretic hormone secretion, ab-normal
depth of sleep, genetic predisposition and developmenta delay. Evidence has
also been found for sympathetic hyperactivity and delayed organ maturation as
seen by delay in ossification.
Obstructive lesions of the urinary outflow tract,
which can cause urinary tract infection (UTI) as well as enuresis, have been
thought to be important, with a high prevalence of such abnormalities seen in
enuretic children referred to urologic clinics. This degree of association is not
seen at less specialized pediatric centers, however, and most studies linking
urinary outflow obstruction to enuresis are methodologically flawed (Shaffer et al., 1979). Structural causes for
enuresis should be considered the exception rather than the rule.
UTI has been found to occur frequently in children,
es-pecially girls and a large proportion (85%) of them have been shown to have
nocturnal enuresis. Also, in 10% of bedwetting girls, urinalysis results show
evidence of bacterial infection. The consensus is that as treating the
infection rarely stops the bedwet-ting, UTI is probably a result rather than a
cause of enuresis.
The concept that children with enuresis have low
functional bladder capacities has been widely promoted. Shaffer and colleagues (1984)
found a functional bladder capacity one standard deviation lower than expected
in 55% of a sample of enuretic children in school clinics. Although low
functional capacity may predispose the child to enuresis, successful behavioral
treatment does not appear to increase that capacity, rather the sensation of a
full (small) bladder promotes waking to pass urine so that enuresis does not
occur. Re-duction of nocturnal secretion of antidiuretic hormone (ADH) has been
described in a small number of children with enuresis, causing excessive
amounts of dilute urine to be produced during the night and overwhelming
bladder capacity. Several mechanisms are asso-ciated with enuresis, including
increased nocturnal urine volume, small nocturnal functional bladder capacity,
increased spontane-ous bladder contractions, and the inability to arouse to the
stimulus of a large and/or contracting bladder. This may identify two main
groups of children with enuresis: those who demonstrate nocturnal spontaneous
bladder contractions (detrusor dependent enuresis) and those with nocturnal
polyuria (volume dependent enuresis).
Approximately 70% of children with nocturnal
enuresis have a first-degree relative who also has or has had nocturnal
enuresis. Twin studies have shown greater monozygotic (68%) than dizygotic
(36%) concordance. An association between enu-resis and early delays in motor,
language and social development has been noted in both prospective community
samples and a large retrospective study of clinical subjects (Steinhausen and
Gobel, 1989). Genetic factors are probably the most important in the etiology
of nocturnal enuresis but somatic and psychosocial environmental factors have a
major modulatory effect. Most com-monly, nocturnal enuresis is inherited via an
autosomal dominant mode of transmission with high penetrance (90%). However, a
third of all cases are sporadic. Four gene loci associated with noc-turnal
enuresis have been identified but the existence of others is presumed (locus
heterogeneity). Other psychosocial correlates described include delayed toilet
training, low socioeconomic class, stress events and other child psychiatric
disorders. Stress events seem to be more clearly associated with secondary
enu-resis. Reported events include the birth of a younger, early
hospi-talizations and head injury (Chadwick, 1985).
Psychiatric disorder occurs more frequently in
enuretic children than in other children, although no specific types have been
identified (Mikkelsen and Rapoport, 1980). The relative fre-quency of disorder
ranges from two to six times that in the gen-eral population and is more
frequent in girls, in children who also have diurnal enuresis and in children
with secondary enuresis.
There is little evidence that enuresis is a symptom
of underlying disorder because psychotherapy is ineffective in reducing
enuresis, anxiolytic drugs have no antienuretic effect, tricyclic
antidepressants exert their therapeutic effect independent of the child’s mood,
and purely symptomatic therapies, such as the bell and pad, are equally
effective in disturbed and nondisturbed children. A further explanation for the
association is that enuresis, a distressing and stigmatizing affliction, may
cause the psychiatric disorder. However, although some studies have shown that
enuretic children who undergo treatment become happier and have greater
self-esteem, other studies show that psychiatric symptoms do not appear to
lessen in children who are successful with a night alarm. A final possibility
is that enuresis and psychiatric disorder are both the result of joint
etiological factors such as low socioeconomic status, institutional care, large
sibships, parental delinquency, and early and repeated disruptions of maternal
care. Shared biological factors may also be important in that delayed motor,
speech and pubertal development, already shown to be associated with enuresis,
have proven to be more frequent in disturbed enuretic children than in those
without psychiatric disorder.
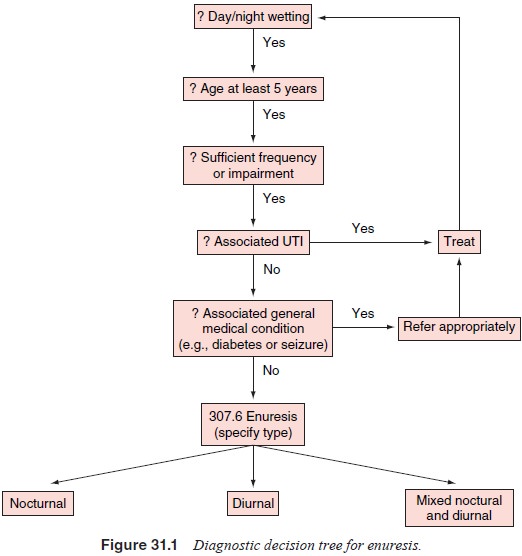
Diagnosis and Differential Diagnosis
The presence or absence of conditions often seen in
association with enuresis should be assessed and ruled out as appropriate
(Figure 31.1). Other causes of nocturnal incontinence should be excluded, for
example, those leading to polyuria (diabetes mel-litus, renal disease, diabetes
insipidus) and, rarely, nocturnal epilepsy.
Assessment
History
Information on the frequency, periodicity and duration of symp-toms is needed to make the diagnosis and distinguish functional enuresis from sporadic seizure-associated enuresis. If there is diurnal enuresis, an additional treatment plan is required. A fam-ily history of enuresis increases the likelihood of a diagnosis of functional enuresis and may explain a later age at which chil-dren are presented for treatment. Projective identification by the affected parent–whereby the parent does not separate feelings about himself having the diagnosis and the current experience of the affected child–may further hinder treatment. For subjects with secondary enuresis, precipitating factors should be elicited, although such efforts often represent an attempt to assign mean-ing after the event.
Questions that are useful in obtaining information
for treat-ment planning include “Why is this a problem?” and “Why does this
need treatment now?” because these factors may influence the choice of
treatment (is a rapid effect needed?) or point to other pressures or
restrictions on therapy. It is important to inquire about previous management
strategies used at home, for example, fluid restriction, nightlifting (getting
the child out of bed to take to the toilet in an often semi-asleep state),
rewards and punish-ments. Parents often come with the assertion that they have
tried everything and that nothing has helped. Examining the reasons for failure
of simple strategies is useful for ensuring that more sophisticated treatments
do not befall the same fate. There is little evidence that fluid restriction is
useful, although nightlifting may be beneficial for the large number of
children who never reach professional attention. Rewards are usually material
and are given only for unreasonably high performance levels, with the delay
be-tween action and reward being too long. Physical punishment and verbal
chastisements, ineffective at best, may well maintain the enuresis. Punishment
is often too harsh and tends to be applied inconsistently depending on parental
mood. If specific treatments have been prescribed, either behavioral or
pharmacological, it is important to discover the reasons they may have failed.
Mental Status Examination
The child’s views and any misconceptions that he or
she may have about the enuresis, its causes and its treatment should be fully
explored. Asking the child for three wishes may help determine whether the
enuresis is a concern to the child. This may unmask marked embarrassment or
guilt from behind a facade of denial about the problem and can be educational
for parents who believe their children could stop wetting “if only they wanted
to or tried harder”. Pictures drawn by the child that describe how the child
views himself or herself when enuresis is a problem and when it is not appropriate
for younger children and can graphically illus-trate the misery experienced by
children with enuresis.
Physical Examination
All children should have a routine physical
examination, with par-ticular emphasis placed on detection of congenital
malformations indicative of urogenital abnormalities. A midstream specimen of
urine should be examined for the presence of infection. Radio-logical or
further medical investigation is indicated only in the presence of infected
urine, enuresis with symptoms suggestive of recurrent UTI (frequency, urgency
and dysuria), or polyuria.
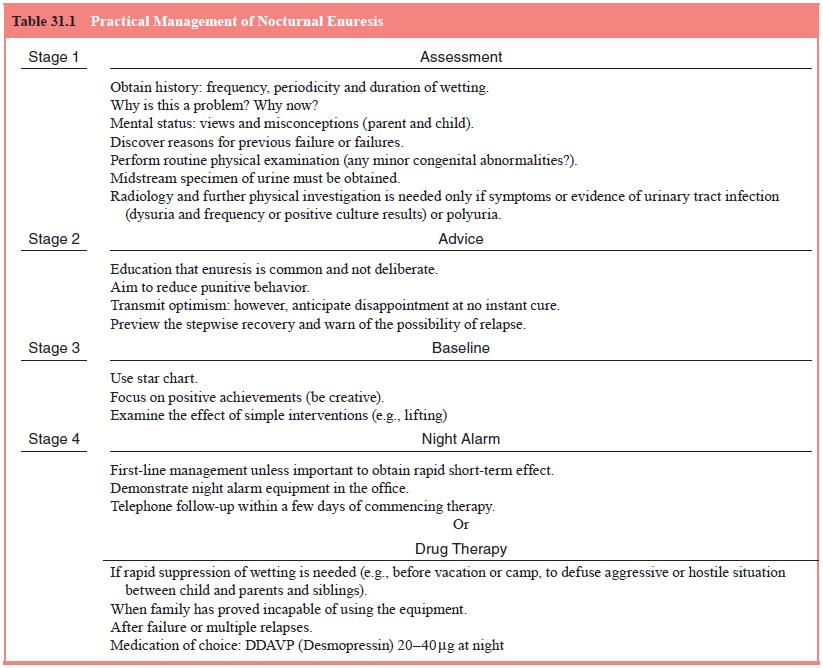
Treatment
Practical management for nocturnal enuresis is
presented in Table 31.1.
The overall goals of treatment can depend on the
reason for referral. Commonly, the child is brought to the physician before
some planned activity, for example, a family vacation or a trip to camp, and
the need is for a rapid (e.g., pharmacological) short-term therapy. A gradual
behavioral approach would not likely meet with much approval even though it may
offer a chance for a permanent cessation of wetting.
Standard Treatment
About 10% of children have a reduction in the
number of wet nights after a single visit to a clinician in which the only
interven-tion was the recording of baseline wetting frequency and simple
reassurance. Such reassurance should make clear that enuresis is a biological
condition that is made worse by stress and that may be associated in a
noncausal way with other psychiatric disor-ders. Younger children can be told
that their problem is shared by many others of the same age. The excellent
prognosis for patients who comply with therapy should be stressed. Recording
the fre-quency of enuresis can be achieved by using a simple star chart. This
is most effective if performed by the child, who records each dry night with a
star. The completed chart is then shown to the parents on a daily basis, and
they can provide appropriate praise and reinforcement.
Waking and Fluid Restriction
Although systematic studies have failed to show any
effect of these interventions with enuretic inpatients, it may be that these
strategies work for the majority of enuretic children who are not referred for
treatment.
Surgery
Based on the premise that enuresis is causally
associated with outflow tract obstruction, various surgical procedures have
been advocated, for example, urethral dilatation, meatotomy, cystoplasty and
bladder neck repair. This cannot be supported because, in addition to the
dubious concept of outflow tract ob-struction per se, the surgery does not
alter the urodynamics of the bladder. Reported positive treatment effects are
slight (no controlled studies exist), and there remains a significant
poten-tial for adverse effects (urinary incontinence, epididymitis and aspermia).
Pharmacotherapy
Although it has been repeatedly demonstrated that
temporary suppression rather than cure of enuresis is the usual outcome of drug
therapy, it remains the most widely prescribed treatment in the USA. Four
classes of drugs have principally been employed: synthetic antidiuretic
hormones, tricyclic antidepressants, stimu-lants and anticholinergic agents.
Synthetic
Antidiuretic Hormone A number of randomized double-blind placebo-controlled trials (RCT) have shown that the
synthetic vasopeptide DDAVP (desmopressin) is effective in enuresis. The drug
is usually administered intranasally, although oral preparations of equal
efficacy have been developed (equiva-lent oral dose is 10 times the intranasal
dose). Almost 50% of children are able to stop wetting completely with a single
nightly dose of 20 to 40 μg of
DDAVP given intranasally. A further 40% are afforded a significant reduction in
the frequency of enuresis with this treatment. As with tricyclic
antidepressants, however, when treatment is stopped, the vast majority of
individuals re-lapse. Side effects of this medication include nasal pain and
con gestion, headache, nausea and abdominal pain. Serious problems of water
intoxication, hyponatremia and seizures are rare. It is important to be aware
that intranasal absorption is reduced when the patient has a cold or allergic
rhinitis. The mode of action of desmopressin is unknown. It may reduce the
production of night-time urine to an amount less than the (low) functional
volume of the enuretic bladder, thereby eliminating the urge to mictur-ate.
With regard to identifying those most likely to respond to DDAVP treatment, it
has been found that those most likely to be permanently dry are infrequent
wetting older children who respond to lower dose (20 μg) desmopressin.
Tricyclic
Antidepressants The short-term effectiveness of imipramine and other related antidepressants has also been
demonstrated via many RCTs. Imipramine reduces the fre-quency of enuresis in
about 85% of bed-wetters and eliminates enuresis in about 30% of these
individuals. Night-time doses of 1 to 2.5 mg/kg are usually effective and a
therapeutic ef-fect is usually evident in the first week of treatment. Relapse
after withdrawal of medication is almost inevitable, so that 3 months after the
cessation of tricyclic antidepressants, nearly all patients will again have
enuresis at pretreatment levels. Side effects are common and include dry mouth,
dizziness, postural hypotension, headache and constipation. Toxicity after
acci-dental ingestion or overdose is a serious consideration, caus-ing cardiac
effects, including arrhythmias and conduction de-fects, convulsions,
hallucinations and ataxia. Concern has been expressed about the possibility of
sudden death (presumably caused by arrhythmia) in children taking tricyclic
drugs. The mode of action for tricyclic antidepressants is unclear, although
one observation is that tricyclic agents seem to increase func-tional bladder
volumes possibly resulting from noradrenergic reuptake inhibition.
Stimulant
Medication Sympathomimetic stimulants such as dexamphetamine have been used to
reduce the depth of sleep in children with enuresis; but because there is no
evidence that enu-resis is related to abnormally deep sleep, their lack of
effective-ness in stopping bed-wetting is no surprise. Used in combination with
behavioral therapy, there is some evidence that stimulants can accentuate the
learning of nocturnal continence.
Anticholinergic
Drugs Drugs such as propantheline, oxybu-tynin and terodiline can reduce the
frequency of voiding in indi-viduals with neurogenic bladders, reduce urgency
and increase functional bladder capacity. There is no evidence, however, that
these anticholinergic drugs are effective in bed-wetting, although they may
have a role in diurnal enuresis. Side effects are fre-quent and include dry
mouth, blurred vision, headache, nausea and constipation.
Psychosocial Treatments
The night alarm was first used in children with
enuresis in the 1930s. This system used two electrodes separated by a device
(e.g., bedding) connected to an alarm. When the child wet the bed, the urine
completed the electrical circuit, sounded the alarm and the child awoke. All
current night alarm systems are merely refinements on this original design. A
vibrating pad beneath the pillow can be used instead of a bell or buzzer, or
the electrodes can be incorporated into a single unit or can be mini-aturized
so that they can be attached to night (or day) clothing. With treatment, full
cessation of enuresis can be expected in 80% of cases. Reported cure rates
(defined as a minimum of 14 consecutive dry nights) have ranged from 50 to
100%. The main problem with this form of enuretic treatment, however, is that
cure is usually achieved only within the second month of treatment. This factor
may influence clinicians to prescribe pharmacological treatments that, although
more immediately gratifying, do not offer any real prospect of cure. It has
been suggested that adjuvant therapy with methamphetamine or desmopressin will
reduce the amount of time before continence is achieved. Using a louder
auditory stimulus or using the body-worn alarm may also improve the speed of
treatment response. Factors associated with delayed acquisition of continence
in-clude failure of the child to wake with the alarm, maternal anxi-ety and a
disturbed home environment, although no influence has been seen regarding the
age of the child or the initial wet-ting frequency.
A further consequence of the delayed response to a
night alarm is premature termination occurring in as many as 48% of cases and
is more common in families that have made little pre-vious effort to treat the
problem, in families that are negative or intolerant of bed-wetting, and in
children who have other behav-ioral problems. Compliance-reducing factors also
include failure to understand or follow the instructions, failure of the child
to awaken, and frequent false alarms. The only reported side effect of
treatment with the night alarm is “buzzer ulcers” caused by the child lying in
a pool of ionized urine. This problem has been eliminated with modern
transistorized alarms that do not employ a continuous, relatively high voltage
across the electrodes to de-tect enuresis.
Relapse after successful treatment, if it occurs,
will usually take place within the first 6 months after cessation of treatment.
It is reported that approximately one-third of chil-dren relapse; however, no
clear predictors of relapse have been identified.
Table 31.2 presents various remedies for night
alarm problems.

Ultrasonic Bladder Volume Alarm
Although the traditional enuresis alarm has good
potential for a permanent cure, the child is mostly wet during treatment.
Fur-thermore, the moisture alarm requires that the child make the somewhat
remote association between the alarm event and a full bladder after the bladder
has emptied. In an exploratory study (Pretlow,
1999), a new approach to treating nocturnal enuresis was investigated
using a miniature bladder volume measurement instrument during sleep. In this,
an alarm sounded when bladder volume reached 80% of the typical enuretic
volume. Two groups were studied. Group 1 used the night-time device alone;
group 2, in addition, had supplementary daytime bladder retention train-ing
(aiming to increase functional capacity). In groups 1 and 2 the mean dryness
rate before study initiation versus during the study was 32.9 and 9.3% versus
88.7 and 82.1%, respectively. Night-time bladder capacity increased 69% in
group 1 and 78% in group 2, while the cure rate was 55% (mean treatment pe-riod
10.5 months) and 60% (mean treatment period 7.2 months), respectively.
Acupuncture
The efficacy of traditional Chinese acupuncture has
been studied (Serel et al., 2001) in
a small (n 5 50)
clinical sample. It was reported that within 6 months, 86% of patients were
completely dry and a further 10% of patients were dry on at least 80% of
nights. Relapse rates appeared better than with
psychopharma-cologic agents.
Summary
There were approximately 22 randomized trials
conducted be-tween 1985 and 1997 involving 1100 children treated
pharma-cologically or behaviorally for primary nocturnal enuresis. The quality
of many of these trials is poor with very few trials com-paring drugs with each
other, or drugs with alarms or other be-havioral interventions, and few having
adequate follow-up pe-riods. Desmopressin and tricyclics appeared equally effective
while on treatment, but this effect was not sustained after treat-ment stopped.
It is clear that further comparisons between drug and behavioral treatments are
needed, and should include relapse rates after treatment is finished.
Assessment and Management of Diurnal Enuresis
Daytime enuresis, although it can occur together
with night-time enuresis, has a different pattern of associations and responds
to different methods of treatment. It is much more likely to be associated with
urinary tract abnormalities and to be comorbid with other psychiatric
disorders. As a result, a more detailed and focused medical and psychiatric
evaluation is indicated. Urine should be checked repeatedly for infection, and
the threshold for ordering ultrasonographical visualization of the urological
sys-tem should be low. The history may make it apparent that the daytime
wetting is situation specific. For example, school-based enuresis in a child
who is too timid to ask to use the bathroom could be alleviated by the teacher’s
tactfully reminding the child to go to the bathroom at regular intervals.
Observation
of children with diurnal enuresis has estab-lished that they do experience an
urge to pass urine before mictu-rition but that either this urge is ignored or
the warning comes too late to be of any use because of an “irritable bladder”.
Therefore, treatment strategies are based on establishing a pattern of
toilet-ing before the times that diurnal enuresis is likely to occur (usu-ally
between 12 noon and 5 pm) and using positive reinforcement to promote regular
use of the bathroom.
Portable systems that can be worn on the body and
use a sensor in the underwear as well as an alarm that can be worn on the wrist
have been developed. Studies have shown no signifi-cant differences between the
wetness alarm and the simple timed alarm. The easiest therapeutic alternative,
therefore, is to buy the child a digital watch with a countdown alarm timer.
Unlike nocturnal enuresis, drug treatment with
tricyclic antidepressants such as imipramine is ineffective, whereas the use of
anticholinergic agents such as oxybutynin and terodiline shows a therapeutic
impact on the frequency of daytime enuresis.
Related Topics