Chapter: Chemistry : Engineering Materials
Engineering Materials
ENGINEERING MATERIALS
1 Introduction
2 Abrasives
2.1 Properties of Abrasives
2.2 Classification of Abrasives
2.3 Applications of Abrasives
3 Refractories
3.1 Characteristics of Refractories
3.2 Classification of Refractories
3.3 Properties of Refractories
3.4 Manufacturing of Refractories
4 Portland Cement
4.1 Chemical composition of Portland cement
4.2 Manufacturing methods
4.3 Setting and hardening
4.4 Other types of cements
5 Glass
5.1 Properties of glass
5.2 Manufacturing of glass
5.3 Types and uses of glass
6 Glossary
1 INTRODUCTION
Materials which are used in manufacturing of wools
and equipments and in construction of buildings, where very specific
requirements are needed are called engineering materials. These include cement,
refractories, abrasives, lubricants, etc,
2 ABRASIVES
Abrasives are very hard substances used for
grinding, shaping and polishing other materials
2.1 PROPERTIES
Have very high melting point
Chemically inert
High abrasive power (ability to scratch away pr
sharp other materials)
Sometimes hard and brittle or soft and flexible
2.2CLASSIFICATION OF ABRASIVES – TYPES &
2.3 APPLICATIONS OF ABRSASIVES
natural abrasives – Eg. Diamond, corundum
synthetic abrasives – Eg. carborundum, norbide
Hardness is measured in terms of moh‟s scale.
Diamond is taken as the reference and hardness of
other materials are determined
abrasives with Mohr‟s scale
1-4 are called soft abrasives
NATURAL ABRASIVES
Diamond:
Purest crystalline carbon - Hardest natural
substance
Mohr‟s scale value is 10 -Superior
chemical inertness
Used in grinding wheels, drilling tools, cutting
glasses, etc
Corundum
Pure
crystalline form of alumina - Mohr‟s scale value is 9 - Used in
grinding glass, gems etc.
Emery
55-75%
alumina, 20-40% magnetite, 12% others - Black and opaque
-Mho‟s scale
value is 8 - Used for making abrasive paper, abrasive cloth, etc.
Quartz
Pure
silicone - Mohr‟s scale value is 7 - Used in painting industries
Garnet
Trisilicates
of alumina, magnetite and Fe oxide used for the manufacture of abrasive paper
and cloth.
ARTIFICIAL ABRASIVES
Silicon Carbide (SiC)
Manufacture
Silicon Carbide is manufactured by heating sand
(60%)and coke (40%) with some saw dust and a little salt in an electric furnace
to about 1500°C
SiO2 + 3C gives SiC + 2CO
The silicon carbide removed from the furnaces, is
then mixed with bonding agent(clay, silicon nitride) and than shaped, dried and
fired.
Properties
Silicon carbide possesses a high thermal
conductivity, low expansion and high resistance
to
abrasion and spalling.
They are mechanically strong. Mohr‟s scale
value is 9.
Bear very high temp. 1650°C
Has thermal conductivity between metals and
ceramics –
They are
electrically intermediate between conductors and insulators.
Uses
Silicon
carbide are used as heating elements in furnaces in the form of rods or bars.
They are also used for partition wall of chamber
kilns, coke ovens, muffle furnaces and floors of heat treatment furnaces.
Sic
bonded with tar are excellent for making high conductivity crucible.
Norbide or Boran Carbide (B4C)
Manufacture
It is
prepared by heating a mixture of boran oxide (B2O3) and coke in an electric
furnace to about 2700°C
B2O3
+7C give B4C + 6CO
Properties
Its hardness is 9 on Mohr‟s scale.
It is light weight and black colored compound.
It is highly resistant to chemical attack and
erosion.
It resists oxidation much better than diamond.
Uses
It is used as hard materials for making grinding
dies, and for cutting and sharpening hard high speed tools.
It is used to prepare scratch and wear resistant
coating.
3 REFRACTORIES
Materials that can withstand high temp without
softening and deformation in their shape.Used for the construction of furnaces,
converters, kilns, crucibles, ladles etc.
3.1CHARACTERISTICS
Infusible at operating temp.
Chemically inert towards corrosive gases, liquids
etc. Should not suffer change in size at operating temp. Should have high
refractoriness
Should have high load bearing capacity at operating
temp.
3.2 CLASSIFICATION
Based on
chemical nature
· Acidic refractories – Eg. Silica and Alumina
Basic refractories – Eg. Magnesite and Dolomite
Neutral refractories – Eg. Graphite and Carborundum
Based on refractoriness
Low heat duty refractories
Intermediate heat duty
refractories
High heat duty refractories
Super heat duty refractories
3.3 PROPERTIES of Refractoriness
It is the ability to withstand very high temp.
without softening or deformation under particular service condition. Since most
of the refractories are mixtures of several metallic oxides, they do not have a
sharp melting point. So the refractoriness of a refractory is generally
measured as the softening temperature and is expressed in terms of pyrometric
cone equivalent.(PCE). Pyrometric cone equivalent is the number which
represents the softening temperature of a refractory specimen of standard
dimension (38mm height and 19mm triangular base) and composition.
Objectives of PCE test
To
determine the softening temperature of a test refractory material.
To
classify the refractories
To
determine the purity of the refractoreies
To check
whether the refractory can be used at particular servicing temperature.
Refractoriness
is determined by comparing the softening temperature of a test cone with that
of a series of segar cones. Segar cones are pyramid shaped standard refractory
of definite composition and dimensions and hence it has a definite softening
temperature.
A test cone is prepared from a refractory for which
the softening temperature to be determined, as the same dimensions of segar
cones. Then the test cone is placed in electric furnace. The furnace is heated
at a standard rate of100C per minute, during which softening of segar cones
occur along with test cone. The temperature at which the apex of the cone
touches the base is taken as its softening temperature.
RUL – Refractoriness Under Load
The temp.
at which a std dimensioned specimen of a refractory undergoes 10% deformation
with a constant load of 3.5 or 1.75 Kg/cm2 The load bearing capacity
of a refractory can be measured by RUL test. A good refractory should have high
RUL value
Porosity – ratio of pore volume to the bulk volume
P = (W-
D/W- A) X 100
W –
weight of saturated specimen in air
D –
weight of dry specimen
A –
weight of saturated specimen in water
Porosity
reduces strength, corrosion resistance thermal conductivity, thermal spalling
and abrasion resistance
Thermal spalling – property of breaking, cracking or
peeling of refractory material under
high temp. Thermal spalling may be due to rapid change in temp. or slag
penetration. A good refractory should show good resistance to thermal spalling
Dimensional stability
Resistance of refractory to any volume change when
exposed to high temp. over a prolonged time. Refractories may undergo
reversible or irreversible dimensional changes A good refractory should show
minimum level of reversible dimensional changes with temp.
3.4 MANUFACTURING OF REFRACTORIES
ALUMINA BRICKS
Contain
50% of aluminium oxide Manufacture
Calcined bauxite, silica and grog (calcined fire
clay) are ground well and mixed with water. The pasty mass is converted into
bricks by mechanical pressing or slip casting.The bricks are dried and fired at
about 1200 to 14000 C for 6-8 days
MAGNESITE BRICKS
Contain
maximum Magnesium oxide
Manufacture
Calcined
magnesite, magnesia or iron oxide are ground well and mixed with water. The
pasty mass is converted into bricks by mechanical pressing or slip casting. The
bricks are dried and fired at about 15000 C for 8 hours then cooled slowly
ZIRCONIA BRICKS
Contain
zirconite
Manufacture
Zirconite
mineral, colloidal zirconia or alumina are ground well and mixed with water and
made into bricks. Small amount of MgO or CaO is added as stabilizer. The bricks
are dried and fired at about 17000 C
4 PORTLAND CEMENT
It is
defined as an extremely finely ground product.
It is obtained by heating a mixture of argillaceous
(clay containing ) and calcareous (lime containing ) raw materials to about 1500
c. It is then mixed with gypsum to increase the quick setting and hardening
property.
4.1 CHEMICAL COMPOSITION OF PORTLAND CEMENT
3CaO.SiO2
- Tri calcium Silicate 3CaO.Al2O3 - Tri calcium
Aluminate
4CaO.Al2O3.Fe2O3
- Tetra calcium alumino Ferrate
4.2 MANUFACTURE OF PORTLAND
CEMENT
Raw
materials :
(i)
Calcareous materials , CaO Ex:
Limestone, chalk.
(ii) Argillaceous materials, Al2O3
and SiO2 Ex: clay,
slate etc
Powdered coal (or) fuel oil.
Gypsum (CaSo4.2H2O)
Manufacture of Portland cement involves the
following steps:
Mixing of raw materials
Burning
Grinding
Storage and Packing
(a) Dry
Process (b) Wet Process
Dry Process: In dry process, the raw materials like
limestone and clay(3:1) are dried, and mixed in definite proportions
Wet
process : In wet process, the raw materials in definite proportions are finely
ground with water and the slurry ( past like) is fed at the top of the rotary
kiln.
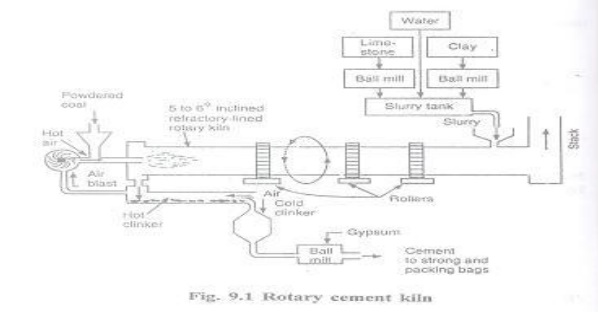
(II) Burning
The burning process is usually done in rotary kiln
which is a long horizontal steel cylinder coated with refractory bricks and
capable of rotating at 1 rpm 9 Revolution per minute) . The rotary kiln is set
at a slight inclination of about 5-60 in order to allow the raw
materials fed at one end to travel slowly to the firing and discharge exit end.
The
slurry of raw materials is allowed to enter from the top end of the rotary
kiln. Simultaneously the burning fuel ( like powdered coal or oil) and air are
introduced from the lower end of kiln . The slurry gradually comes down in the
kiln into the different zones ( Drying Zone at 400o :Calcination
zone at 700 -1000 o C and clinkering zone at 1250-1500 o
C of increasing temperatures.
Drying
Zone: The upper part of the rotary kiln is known as drying zone ,where the
temperature is about 400 o C . Due to the presence of hot gases in
this zone, water is evaporated from the slurry.
Calcinations
zone: The middle part of the rotary kiln is known as calcining zone where the
temperature ranges from 700 -1000 o C. In this zone lime stone is
decomposed into CaO and CO2.
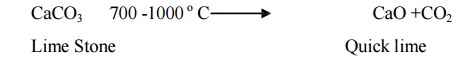
(c) Clinkering Zone : The lowest part of the zone
is called as clinkering zone, where the temperature is maintained about 1250-1500
o C. In this zone lime reacts with clay ( Containing Al2O3,
Fe2O3 and SiO2) and forms aluminates and silicates

The
mixture is then finely powdered and fed into the top of the rotary kiln.

Cooling : the hot clinker is cooled with
atmospheric air and the hot air thus produced is used for drying the coal
before grinding.
Grinding : The cooled clinker is then finely
pulverized with 2-6% gypsum acts as a retarding agent for quick setting of
cement.
Storage and Packing: The cement coming out from the
grinding mills is stored in a concrete storage silos. Then the cement is packed
in jute bags by automatic machines. Each bag contains 50kgs of cement.
PROPERTIES
4.3 SETTING AND HARDENING OF
CEMENT:
When the cement is mixed with water, hydration and
hydrolysis of cement begin, resulting in the formation of gel and crystalline
products.
Setting: It is defined as the stiffening of the
original plastic mass, due to initial gel formation. Hardening: It is defined
as the development of strength, due to crystallization.
Chemical reactions involved in
setting and hardening of cement:
When water is mixed with cement , hydration of
tricalcium aluminate occurs rapidly and the paste becomes quite hard within a
short time. This process is known as initial setting of cement.
3CaO.Al2O3 +6H2O-------3CaO.Al2O3.6H2O
Role of gypsum in cement:
(i) In
initial setting process gypsum is added during grinding of cement clinkers to
retard the rapid hydration
of C3A. Gypsum reacts with C3A
to form insoluble calcium sulphoaluminate complex. C3A + 3CaSO4.2H2O--------C3A.3CaSO4.2H2O
After the
hydration of C3A,C3S begins to hydrate to give
tobermonite gel and crystalline Ca(OH)2. The hydration of C3S
takes place within 7days.
2(3CaO.SiO2) + 6H2O---------
3CaO.2SiO2.3H2O +
3Ca(OH)2 + 500kj/kg
Dicalcium
silicate reacts with water slowly and gets finished 7-28days.
2(2CaO.SiO2) + 4 H2O -------- 3CaO.2SiO2.3H2O +Ca(OH) 2 + 250kj/kg
Hydration
of tetra calcium aluminoferritetakesplace initially, the hardening takes place
finally through crystallization along with C2 S.
4CaO.Al2O3.Fe2O3 + 7H2O----------3CaO.Al2O3.6H2O(Crystalline)
+ CaO.Fe2O3.H2O(gel) + 420KJ
Thus the
final setting and hardening of cement is due to the formation of tobermonite
gel plus crystallization of Ca(OH)2 and hydrated tricalcium
aluminate.
4.4 OTHER TYPES OF CEMENT -
SPECIAL CEMENT
Water Proof Cement :
It is obtained by adding water proofing agents like
calcium stearate and gypsum with tannic acid to ordinary Portland cement during
grinding.
Functions of water- Proof cement:
Functions
of water- proof cement
To make concrete impervious to water under
pressure.
To resist the adsorption of water.
White cement or White Portland
cement
It is obtained by heating the raw materials free
from iron oxides. It is white in color due to the absence of ferric oxide.
It issued for making tiles, mosaic works with some
coloring agents like yellow ochre, Venetian red etc. It is used for repairing
and joining marble pillars and blocks.
5 GLASS
Glass is
an amorphous, hard brittle, transparent, super cooled liquid of infinite
viscosity.
Glass may
be represented as xR2O.yMO.6SiO2
5.1 GENERAL PROPERTIES OF GLASS:
It is amorphous.
It is very brittle.
It softens on heating.
It has no definite melting point.
It is affected by alkalis.
It is a good electrical insulator.
It can absorb, reflect or transmit light.
It is not affected by air water, acids and chemical
agents.
5.2 MANUFACTURE OF GLASS
Melting :
The raw materials in proper proportions are mixed
and finely powdered.This homogeneous mixture is known as BATCH is fused with
some broken glass called CULLET in the pot of the furnace.The furnace is heated
by burning producer gas and air mixture over the charge. The cullet melts at a
low temp and assists in melting the rest of the charge.
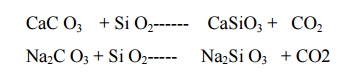
Forming and Shaping
The molten glass is then worked into articles of
desired shapes by either blowing or moulding or pressing between rollers.
Annealing:
Glass
articles are then allowed to cool gradually to room temperature. Suddencooling
must be avoided, because cracking occurs.Longer the annealing period, the
better is the quality of the glass.
Finishing:
All glass
articles after annealing, are subjected to finishing processes such as
(a) Cleaning (b) grinding (c)
polishing (d) cutting (e) sand
blasting
5.3 TYPES AND USES OF GLASSES
1.Soda-lime or soda glass
(i) Raw materials: Silica , calcium carbonate and
soda ash (ii) Composition: Na2O. CaO. 6SiO2
Properties
They are low in cost.
They are resistant to water
They are attacked by common reagents like acids.
They melt easily‟
Potash
lime or
Hard glass
Raw materials :
Silica, CaCO3, K2CO3
Composition:
K2O.
CaO.6SiO2
Properties:
(a) They
have high melting point.
They do not fuse easily.
They are less acted upon acids alkalis, solvents.
Uses: Used for manufacturing combustion tubes,
chemical apparatus
3. Lead glass or Flint glass
(i) Raw
materials: Lead
oxide,
silicva, K2O
(ii) Composition:
K2O.PbO.6SiO2
Properties:
It is bright and lustrous
It has high specific gravity. (3 to 3.3)
It is more expensive to manufacture.
It has a lower softening temperature than soda
glass.
It has higher refractive index.
Uses: (a) These are used for high quality tablewares.
They are used in neon sign tubings, optical lenses,
electrical insulators, cathode ray tube.
Potash
lime or
Hard glass
Raw materials :
Silica, CaCO3, K2CO3
Composition:
K2O.
CaO.6SiO2
(iii)
Properties:
(a) They
have high melting point.
They do not fuse easily.
They are less acted upon acids alkalis, solvents.
Uses: Used for manufacturing combustion tubes,
chemical apparatus
Lead
glass or Flint glass
Raw materials:
Lead oxide, silicva, K2O
Composition:
K2O.PbO.6SiO2
Properties:
It is bright and lustrous
It has high specific gravity. (3 to 3.3)
It is more expensive to manufacture.
It has a lower softening temperature than soda
glass.
It has higher refractive index.
Uses: (a) These are used for high quality tablewares.
(b) They
are used in neon sign tubings, optical lenses, electrical insulators, cathode
ray tube.
4. Borosilicate glass or Pyrex
glass or Jena glass
(i)Raw materials: Silica, borax with small amount
of alumina and some oxides. (ii) Composition : SiO2 (80.5%); B2O3
(13%)
Al2O3 (3%) K2O (3%) Na2O (0.5%)
Properties:
It possess low thermal coefficient of expansion and
high chemical resistance. (2)It possesses very high softening points and
excellent resistivity.
Uses: It is used in industry for pipe lines for
corrosive liquids, gauge glasses,
5. Alumina silicate glass
Raw
materials: It has 5% or more alumina
(i)Composition: SiO2 Al2O3, B2O3,
MgO, CaO, Na2O K2O
Properties:
They
possess high softening temperature.
Uses:
(a)Used
in high pressure mercury discharge tubes
(b)Chemical
combustion tubes.
6. Optical or Crookes glass
Raw
materials: It contains phosphorus, lead silicate with small amount of cerium
oxide. Properties:
(a) Cerium
oxide present in the glass absorbs uv light,
(b) They have
low melting point.
Uses:
optical glasses are used for making lenses.
7. Glass wool
Glass wool is fibrous wool like material It is
composed of intermingled fine threads or filaments of glass.
Properties: It is a very good heat and fire proof materials
Its electrical conductivity is low.
Uses; It is
used for heat insulation purposes
It is
used for electrical and sound insulation.
6 Glossary
Abrasives
Abrasives are hard substances, used for
polishing, shaping, grinding operations. They are characterized by high melting point, high hardness and
chemically inactive.
Refractories
Refractories are materials that can withstand
high temperatures without softening or deformation
in shape.
Refractoriness
Refractoriness is the ability of a material to
withstand very high temperature without softening
or deformation under particular service condition.
Pyrometric cone equivalent
Pyrometric
cone equivalent is a number which represents the softening temperature of a
refractory specimen of standard dimension ( 38 mm height and 19 mm triangular
base ) and composition.
RUL ( Refractoriness Under Load )
The
temperature at which the refractory deforms by 10% under a load of 3.5kg/cm2
is called RUL (Refractoriness Under
Load)
Porosity
Porosity is defined as the ratio of its
pore volume to the bulk volume
Thermal spalling
Thermal spalling is the property of breaking,
cracking or peeling off a refractory material
under high temperature. A good refractory must show a very good resistance to
thermal spalling.
Calcination
Heating
the ore in absence of air is called Calcination
Cement
Cement is a material with adhesive and
cohesive properties which make it capable of bonding minerals fragments into a compact whole. The name “Portland cement” given originally due
to the resemblance of the colour and quality of the hardened cement to Portland
sonte ( Portland island is England).
Related Topics