Chapter: Chemistry : Engineering Materials
Important Questions and Answers: Engineering Materials
ENGINEERING MATERIALS
01. What are Abrasives? Explain
with classification.
Abrasives
are hard substances used for Grinding, cutting, Shaping, drilling, poslishing
andsharpening operations.
Ex:
Diamond,Talc
02. Explain the properties of
refractories.
Properties : Refractoriness,
RUL, Dimensional stability, Thermal spalling,
Thermal
expansion , porosity.
Refractoriness:
It is ability of a refractory material to withstand
very high temperature without softening or deformation under the working conditions
It is measured by PCE test.
PCE= Pyrometric cone equivalent
P.C.E number and softening temperature of some
refractories are as follows a. Silica
bricks
Alumina bricks
Magnesite bricks
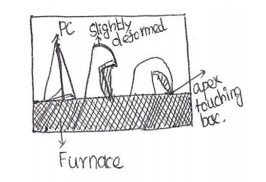
PCE is the number which refer to the
softening temperature of a refractory specimen of standard dimension 38mm
height and 19mm triangular base.
Measurement:
This measurement is called segarcone
test.
A test cone is determined by comparing
its softening temperature with softening temps.of a series of standard
pyrometric cones.
A test cone is prepared from the
sample refactory then it is placed along with the standard segar cone in an
electric furnace and heated at a rate of 10oC per minute.
The temperature at which apex (top) of
the cone touches the base is taken as the softening temperature .the PCE number
of the standard cone which behaves identically is taken as the PCE of the test
sample.

How Alumina, Magnesite and Silicon carbide are
manufactured?
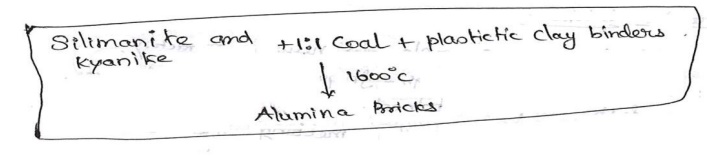
Manufacture
of Alumina bricks:
Alumina
bricks is an example for acidic refractories.
Alumina
bricks are prepared from minerals silimanite and kyanite They are anhydrous
aluminosilicate materials Al2O3.SiO2
This
mineral is fixed with coal in the ratio 1:1 along with plastic clay as binders.
The raw
materials are mixed and moulded into bricks The bricks are then dried and fired
at 1600oc
The final
product material contains 63 % alumina and 34% silica approximately. These are
used in steel industries.
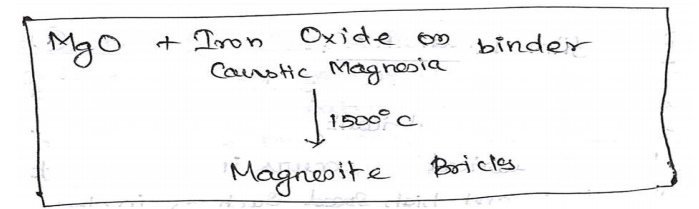
Manufacture
of Magnesite bricks:
Magnesite
bricks are an example for basic refractories
Powdered
caleinedmagnoite (Mgo) is mixed with caustic magnesia or iron oxide, as a
birder with
water
mixed and moulded into bricks.
The bricks
are then dried and fired at 1500oC
The final
product material containsMgO=85%,CaO=2.5% & SiO2=5.5%
There are
used in open hearth furnaces, libing converters and reverberatory furnaces.
Manufacture
of Silicon carbide (SiC): CARBORUNDUM
SiC is an example for very hard synthetic abrasive.
It is a mixture of SiO2 and coke.
Its
hardness is 9.8 on Moh‟s scale.
It is
chemically inert and can withstand high temperature.
Preparation
Or Manufacture:
It is
prepared by heating a mixture of 60% sand (i.e) SiO2 and 40% coke with a small
amount of saw dust and a little salt in an electric furnace to about 1650oC
for 36 hours.
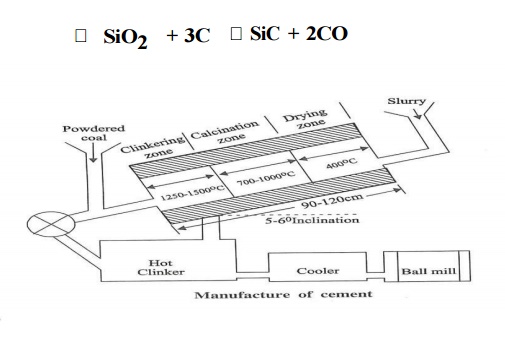
2.
Burning: Dry raw mix / slurry is carried out in rotary kiln.
The Dry
raw mix / slurry is fed into the kiln from upper end and the flame is forced
into the lower end. Due to slope and slow rotation, the material gradually
descends in the klin into different zones of increasing temperatures.
(i). Drying Zone: Upper part of the kiln - About
400oc, water in slurry gets evaporated. (ii). Calcination zone:
Center part of the kiln - About 1000oc, limestone gets decomposed
intoCaO and CO2. CaCO3 ----- CaO + CO2
(iii).
Clinkering Zone: Lowest part of the kiln - About 1350-1500oc,
limestone reacts with clay to form Bogue compounds. C2S,C3S,C3A,C4AF.
The Bogue compounds fuse together to form
small, hard, grayish coloured stone like mass called cement clinkers.
Grinding : The hot clinkers are cooled with
atmospheric air and then pulverized together with 2-3% gypsum in ball mills.
Gypsum act as retarding agent for quick setting cement.
Storage and Packing: The cement coming out of the
grinding mill is stored in a concrete storage silos. Then the cement is packed
in jute bags by automatic machine.
07. Explain the properties of
Portland cement.
Properties:
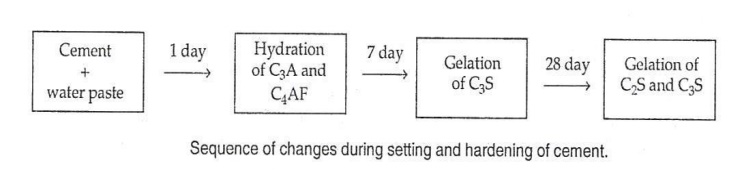
(i). Setting and Hardening of cement. (ii). Heat of Hydration. (i).
Setting
and Hardening of cement.
Setting: It is defined as the stiffening
of the original plastic mass, due to the formation of tobermonite gel.
Hardening: It is
defined as the development of strength due to formation of crystals. When
cement is mixed with water, results formation of gel and crystalline products.
Chemical reactions:
(i).
Flash set – When cement is mixed with water, hydration of C3A takes place and
the paste becomes quite rigid within a short time.(1 Day)
C3A + 6H2O
-- -- > C3A.6H2O
(II).
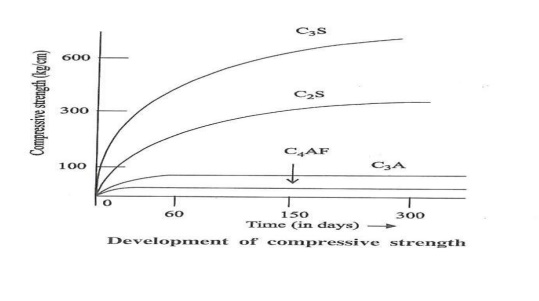
Formation of Tobermonite gel: After the hydration of C3A, C3S begins to hydrate
to give Tobermonite gel and crystalline Ca(OH)2. This is responsible for the
development of initial strength of cement. The hydration of C3S gets completed
within 7 days. It does not contribute much to the strength of cement.
C3S + 6H2O
-- -- > C3S2
.3H2O + 3Ca(OH)2
(III). Dicalcium
silicate (C2S) reacts with water very slowly and gets completed in 7 to 28
days.
2C2S + 4H2O C3S2
.3H2O +Ca(OH)2
The
increase in strength between 7 to 28 days is due to the formation of
tobermonite gel and crystalline Ca(OH)2.
(iv). The
hydration of C4AF takes place initially, the hardening takes place finally
through crystallization along with C2S.
C4AF
+ 7H2O -- -- > C3A.6H2O
Thus the
final setting and hardening of cement is due to the formation of tobermonite
gel plus crystallization of Ca(OH)2 and hydrated tricalcium aluminate.
ii). Heat
of Hydration:
When
water is mixed with Portland cement some amount of heat is liberated due to
hydration and hydrolysis reactions of Bogue compounds. The average quantity of
heat is liberated is 500 kJ/kg.
08. Write short notes on special
cements like waterproof and white cement.
(i). Waterproof cement:
It is obtained by adding water- proof materials
like calcium stearate, aluminium stearate and gypsum with tannic acid to
ordinary Portland cement during grinding.
Functions: (i).
To make concrete impervious to water under pressure. (ii).
To resist
the absorption of water.
Properties:
(i). It
is more expensive than ordinary Portland cement. (ii). It act as pore-blocking
and water- repelling agent.
Uses:
(i). Used
in the construction bridges and structures under water.
(ii).
White Cement:
It is
white in color due to absence of iron compounds.
Such cements are made from raw materials which are
free from iron oxide. Properties:
(i). It
is more expensive than ordinary Portland cement. (ii). It act as pore-blocking
and water- repelling agent.
Uses:
(i).
Repairing and joining marble pillars and blocks, manufacture of tiles and
mosaic
works.
(ii).
Used in the construction bridges and structures under water.
09. Explain the manufacture the
glass.
Raw
materials:
Sodium is soda , Na2CO3 – Soft glass.
Potassium is potash, K2CO3 – Hard
glass. c)
Calcium are limestone, chalk and lime
d) Lead
are litharge, and red lead – Flint glass e) Silica are quartz, white sand.
Zinc is zinc oxide – Heat and shock proof glass
Borate are borax , and boric acid - Heat and shock
proof glass h) Cutlets or pieces of broken glass to increase the fusibility.
4 steps
involved for the manufacturing of glass.
1.
Melting: The raw materials in proper proportions are mixed and finely powdered.
This mixture called batch is fused with some broken glass, called cullet in the
pot of the tank furnace, in which heating is done by burning produces gas and
air mixture over the charge.
Heating
is continued, till the molten mass is free from bubbles and glass-balls, and
then cooled to about 800oC.
2.
Foaming and shaping:
Molten glass is then worked into articles of
desires shapes by either blowing or moulding or pressing between rollers.
3.
Annealing:
Glass articles are then allowed to cool gradually
to room temperature( sudden cooling must be avoided, because cracking occurs ).
The longer the annealing period, the better quality of glass.
4.
Finishing:
All glass articles after annealing are subjected to
finsish processes such as cleaning, grinding, polishing, cutting etc.
Explain
the types and properties and uses of glass.
. Soda
lime or soft glass:
Raw
materials: silica, calcium carbonate and soda ash. Approximate composition:
Na2O.CaO.6SiO2 Properties:
They are low cost.
It is resistant to water.
It is attacked by common reagents like acids.
Uses:
Window glasses, electric bulbs, bottles, jars,
cheaper table wares, where high temperature – resistance and chemical stability
required.
2). Potash lime or Hard glass:
Raw
materials: silica, calcium carbonate and Potassium carbonate.
Approximate
composition: K2O.CaO.6SiO2
Properties:
Possess high melting point so it will not fuse
easily.
Less acted upon by acids, alkali and other solvents
than ordinary glasses.
Uses:
Chemical
apparatus, combustion tubes, which are to be used for heating operations.
3). Lead glass or Flint glass:
Raw
materials: Lead oxide and silica are fused.
Approximate
composition: K2O.PbO.6SiO2
Properties:
a) Lower
softening temperature than soda- glass. b) Higher refractive – index.
c) Has
excellent electrical properties. d) High specific gravity ( 3 to 3.3)
Uses:
High quality table wares, optical purposes(like
lenses), neon sign tubing, cathode ray tubes, electrical insulators and in art
objects.
High lead content glasses are used for extra-dense
optical glasses for windows and shields to protect personnel from x-rays and
gamma rays in medical and atomic energy fields respectively.
4). Boro silicate glass / Pyrex
glass / Jena glass.
Raw
materials: silica, boron with a small amount of alumina and some oxides.
Approximate composition: SiO2(80.5%),B2O3(13%), Al2O3(3%),K2O(3%),Na2O(0.5%)
Properties:
Low thermal efficient of expansion
High chemical resistance.
Very high softening points.
Excellent shock-proof.
Uses:
Used in industry for pipelines for corrosive
liquids, gauge glasses, superior laboratory apparatus, kitchenware, chemical
plants, television tubes, electrical insulators.
5). Aluminosillicate glass
Raw
materials: alumina, silica, boron with a small amount of and some oxides.
Approximate composition: SiO2(55%),Al2O3(23%),B2O3(7%), MgO(9%),CaO(5%),
Na2O+K2O(1%)
Properties:High
softening temperature
Uses:
High-pressure
mercury discharge tubes, chemical combustion tubes, certain domestic
equipments, etc,
6). Glass wool:
It is a
fibrous wool-like material, composed of intermingled fine threads of filaments
of glass. They are completely alkali free. The glass filaments are obtained by
forcing molten glass through small offices. The average diameter of the office
is 0.0005 to 0.007mm. Then the filaments of glass so obtained are thrown over a
rapidly rotating drum to get wool-like materials
Properties:
a). fire-
proof and heat proof material.
b). low
electrical and thermal conductivity.
c).
resistant to water and most of chemicals. d). high tensile strength – 8 times
that of steel.
Uses:
Heat insulation purpose- domestic and industrial
appliances.
Air filters and dust filtering materials.
Insulation of metal pipelines and walls and roofs
of houses.
used in filtration of corrosive liquids like acids.
Manufacture of fibre-glass, by blending with
plastic resins.
Related Topics