Chapter: Essentials of Psychiatry: Electroconvulsive Therapy
Electroconvulsive Therapy(ECT): Treatment
Initiation
of Treatment
Once
informed consent has been obtained, the initiation of treat-ment involves
several decisions. These include selection of ECT device, electrode placement,
dose of electricity, choice of pre-medications and frequency of treatment.
In
choosing electrode placement there are two important factors to consider:
antidepressant efficacy and cognitive side effects. The choices of electrode
placement can be divided into unilateral placement over the nondominant
(generally right) hemisphere and bilateral electrode placement (Figure 74.1),
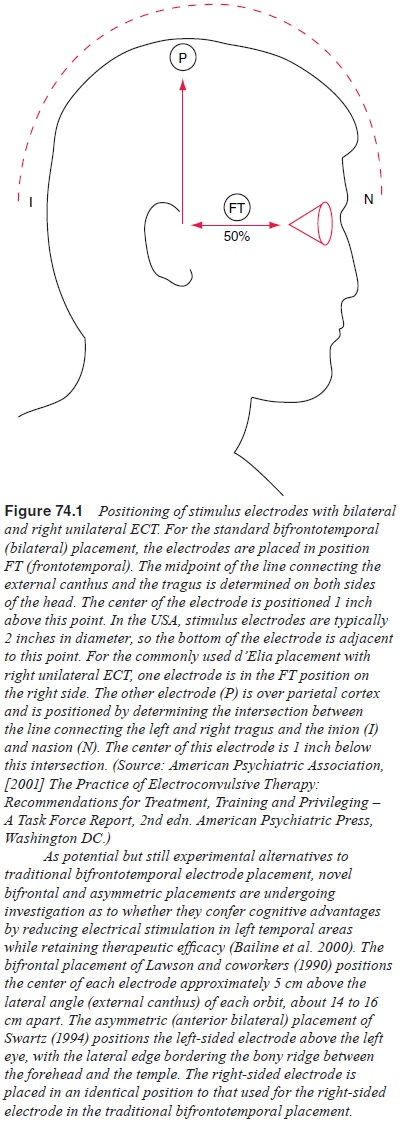
traditionally
bifrontotemporal. The advantage of unilateral placement is that there is less
memory loss and confusion than with bilateral electrode placement (Horne et al., 1985; Weiner et al., 1986; Abrams, 1982). The
disadvantage of unilateral ECT is
that it appears to be less effective when the dose of electricity given is
close to seizure threshold (Sackeim et al.,
1993), and the seizure threshold can vary more than 40-fold from individual to
individual (Sackeim et al., 1987,
1991). With bilateral placement, seizure threshold is less of a concern, and
the degree and speed of response appears greater with (high dose) bilateral
than unilat-eral ECT (Nobler et al.,
1997; Sackeim et al., 2000). Although
bi-frontotemporal placement has been more widely used, a limited number of
studies suggest that bifrontal electrode placement may offer comparable
treatment efficacy with fewer cognitive side ef-fects (Weiner, 1994).
Individuals who are unresponsive to several adequately dosed unilateral
treatments may benefit from a switch to bilateral electrode placement.
From the
start of the treatment procedure (see Table 74.1) EKG, heart rate and blood
pressure are monitored and oxygen saturation is measured via pulse oximetry.
Oxygen by mask is typically administered after the induction of anesthesia and
until the return of spontaneous respiration. Depending upon the pref-erence of
the treatment team, patients may or may not be pre-medicated with an
anticholinergic agent. Atropine and glycopyr-rolate are the agents most
commonly used (Abrams, 1997). The rationale for using these premedications is
twofold. First, they reduce the bradycardia observed immediately after the
delivery of the stimulus, and secondly, they dry secretions during anes-thesia
(Sommer et al., 1989). The decrease
in heart rate initially observed during seizure induction is the result of
increased vagal tone which occurs immediately after the stimulus (Elliot et al., 1982).
The
patient is rendered unconscious with a short-acting general anesthetic.
Methohexital 0.75 to 1.0 mg/kg given intrave-nously is the agent most commonly
used (Folk et al., 2000). Once the
patient is unconscious, a muscle relaxant is administered. In-travenous
succinylcholine 0.5 to 1.0 mg/kg is almost always used for this purpose. The
goal of the muscle relaxant is to dampen the tonic–clonic movements from the
seizure and reduce the risk of musculoskeletal injury (Elliot et al., 1982; Lippmann et al., 1993; Weiner, 1994). The cuff technique (Fink and Johnson, 1982)
may be applied to an ankle or forearm, preventing localized cir-culation of the
muscle relaxant, thereby facilitating monitoring of the motor seizure duration.
The degree of relaxation is some-what dependent upon the preference of the
practitioner; however, when there is a history of skeletal disease the
paralysis should be nearly complete. The fasciculations induced by
succinylcholine can cause myalgias which can be prevented by administration of
a small dose of the nondepolarizing agent, curare, prior to dosing with the
succinylcholine. When curare is used in this manner it is necessary to increase
the succinylcholine dosage by approx-imately 25% to achieve the same level of
muscle relaxation as previously.
When the
patient is unconscious and relaxed, the stimulus is delivered, using the
desired electrode placement. Initially, the jaw will clench as a result of
direct electrical stimulation. The heart rate will slow and the patient will
generally have tonic contraction of the extremities (Elliot et al., 1982). This initial period,
which lasts anywhere from 2 to 5 seconds, is usually followed by a marked
increase in blood pressure and heart rate (McCall, 1993). This is secondary to
a centrally mediated catecholamine surge (Elliot et al., 1982; Swartz, 1993). The extremities change to tonic–clonic
contractions, the intensity of which depends on the degree to which they have
been modified by the muscle relaxant.
During
the treatment, seizure duration should be monitored, if possible, via a one- or
two-channel electroencepha-logram (EEG), an integral component of most modern
ECT de-vices (Stephens et al., 1991).
Combining motor movement timing with EEG monitoring yields the most reliable
seizure duration determinations in the clinical setting (Lippmann et al., 1993). Although dose of
electricity relative to seizure threshold is the important variable, in
general, an adequate seizure is usually between 20 seconds and 2 minutes in
duration.
Once the
seizure terminates, the patient is continuously supported and monitored until
breathing spontaneously and re-sponsive to voice commands, with return of
muscle strength. The patient’s vital signs are monitored every 15 minutes until
stable.
This
process is repeated for an average of 6 to 12 sessions in the treatment of
depression. In the USA, ECT is usually performed three times per week, while in
the UK and Europe twice-a-week schedule is more common. The available data
suggest that the twice-a-week schedule produces an equivalent therapeutic response
with fewer treatments, but the speed of clinical improvement is slower than the
three times per week schedule (Lerer et
al., 1995; Shapira et al., 2000).
On the other hand, the more rapid therapeutic response to thrice weekly ECT is
accompanied by greater cognitive adverse effects than those associated with the
slower rate of treatments (Lerer et al.,
1995; Shapira et al., 2000).
Regardless
of the treatment schedule, the rate of response will vary for each patient.
Often, the patient’s vegetative symp-toms will respond before the patient feels
subjectively improved.
Adverse Effects
The
potential adverse effects from ECT range from mild com-plications such as
myalgias, to serious events such as fractured bones, to catastrophes such as
death. At present, the risk of seri-ous complication is about 1 in 1000
patients. The risk of death is about 1 in 10 000 patients, which approximates
the risk of general anesthesia for a minor surgical procedure (NIH/NIMH, 1985)
and is actually lower than the spontaneous death rate in the com-munity
(Abrams, 1997).
Cardiac
complications are the most frequent medical side effects associated with ECT.
The arrhythmias range in sever-ity from the common and benign sinus tachycardia
to rare life-threatening or fatal ventricular arrhythmias.
Confusion
and memory loss are also commonly occurring side effects. These adverse effects
are the major factor limiting the use of ECT. Transient confusion occurs
universally as a post-ictal event. Memory disturbance also occurs quite
frequently (Calev, 1994). In general, during the acute course of ECT, both
retrograde and anterograde memory are impaired to some degree (NIH/NIMH, 1985;
Calev, 1994). Retrograde amnesia is gener-ally felt to be more problematic.
After the treatments end, the memory difficulties gradually resolve over the
ensuing weeks to months (Lisanby et al.,
2000). Some patients may have per-manent spottiness in memory for events that
occurred in the weeks to months before, during and following the ECT course.
Rarely, patients have complained of persistent memory difficul-ties severe
enough to interfere with social and/or occupational functioning (NIH/NIMH,
1985). However, the infrequency with which this occurs, and certain technical
factors such as the lack of nondepressed pretreatment memory and other
neuropsychiat-ric measures (Coffey, 1994) has made it difficult to study these
individuals systematically. Subjective impressions of post-ECT memory deficits
appear to correlate more closely with clinical outcome and mood state than with
objective cognitive measures (Prudic et
al., 2000). Although evidence to date points to only a transient and
tolerable degree of cognitive impairment with con-tinuation and maintenance ECT
(Datto et al., 2001) as these
treat-ment strategies continue to play an increasing role in the long-term
treatment of mood disorders, more definitive research on their effects on
memory will help guide clinicians.
Few
formal studies of ECT effects on cognitive function-ing in children and
adolescents have been conducted in the past 50 years.
ECT–Drug Combinations and Interactions
As most
patients referred for ECT already are taking psychotro-pic medications, many
ECT–drug interactions result from the inadvertent or intentional continuation
of preexisting medication regimens with the initiation of convulsive therapy.
Community surveys indicate that fewer than half of patients have discontin-ued
all previous psychotropic medications at the time of ECT (Prudic et al., 2000). While more definitive
research is underway, the American Psychiatric Association Task Force
recommends that, particularly for patients with a history of treatment
resis-tance, “concurrent treatment with an antidepressant medication and ECT
should be considered” (American Psychiatric Associa-tion, 2001).
Continuation and Maintenance Treatment
Among the
unique features of ECT is the time-limited nature of its use in the treatment
of acute episodes of illness. Following completion of an acute treatment
course, ECT is generally terminated abruptly, coincident both with clinical
response and, in many cases, impending inpatient discharge. It is now clearly
established that left untreated after completion of ECT, at least half of
patients will relapse, most within 6 months (American Psychiatric Association,
2001; Sackeim et al., 2001a).
Antidepressant treatment is now used routinely following a course of ECT to
help prevent such relapse. Most contemporary authors adhere to the distinction
between continuation treatment, over
6 months or so, to prevent relapse into the index episode, and maintenance treatment beyond that
point, with the goal of avoiding recurrence, that is a new episode of illness.
Nearly all published data on continuation and maintenance treatment have dealt
with ECT administered for the treatment of depression.
Additional
research is necessary to develop even more effective strategies to prevent
relapse after completion of ECT.
Although the optimal methodology for an extension of the traditional ECT course – including electrode placement, frequency and duration of treatment – is yet to be determined (Scott et al., 1991) it has become the subject of the ongoing NIMH-supported four-site CORE trial (O’Connor et al. 2001). Following an acute course of generally successful bilateral ECT (see earlier), patients in the CORE trial were randomized for the next 6 months to either a weekly to monthly maintenance ECT trial or to an active control pharmacotherapy condition, consisting of the most effective post-ECT medication regimen (combined nortriptyline plus lithium) identified by Sackeim et al., (2001a). Moreover, additional data are required on the risk of cognitive and other adverse effects of continuation and maintenance ECT, and the best means of minimizing untoward effects of this potentially valuable intervention (Fox, 2001). There is general agreement that new written informed consent, beyond that obtained for the acute series of treatments, must be secured for continuation/maintenance ECT. In the event of prolonged maintenance ECT, the American Psychiatric Association (2001) Task Force recommends that the informed consent process be repeated every 6 months.
Treatment Failure
The total
population of patients who are considered ECT treat-ment failures can be
divided into three categories: true nonre-sponders; relative nonresponders for
whom ECT can yet be made to work; and individuals for whom, upon closer
inspection and ex-amination, ECT was not the right treatment choice. Thus, the
first approach to the ECT-resistant patient is to assure that an appro-priately
intensive trial of convulsive therapy has been attempted. Then reassessment,
removal of any obstacles to treatment re-sponsivity and, in most cases, entry
into a treatment-resistant depression algorithm are indicated.
Adequacy of ECT Trial
A course
of eight to 12 bilateral ECT treatments should be com-pleted before any patient
is declared ECT resistant. Patients who fail to respond to several treatments
with unilateral electrode placement should be switched to bilateral ECT and
offered an op-portunity to respond to a full trial of that modality (Delva et al., 2001). The treatment history of
the ECT-refractory patient should be reviewed to ensure that seizures were
generalized and of ad-equate duration, and that in the case of unilateral electrode
place-ment, stimulus intensity was sufficiently above seizure threshold. Some
resistant patients may require additional ECT sessions in order to respond
(Sackeim et al., 1990).
Re-evaluation
Even in
carefully selected patients, lack of response to a course of ECT may occur in
10 to 30% of individuals (NIH/NIMH, 1985). Nonetheless, this degree of
refractoriness should trigger a reassessment of the patient, with confirmation
of the original diagnosis. The additional information learned during a hospital-ization
may enable a more accurate assessment of the chronicity of illness, presence of
medical disease, degree of mood congru-ence of symptoms, vegetative
functioning, mood reactivity, Axis II pathology, alcohol or other substance
abuse, and outstanding psychosocial issues than was available on admission.
Such data may both help explain the lack of response to ECT and open av-enues
to further evaluation or treatment efforts.
Related Topics