Chapter: Obstetrics and Gynecology: Dysmenorrhea and Chronic Pelvic Pain
Dysmenorrhea
DYSMENORRHEA
Primary
and secondary dysmenorrhea are a source of recurrent disability for a
significant number of women in their early re-
productive
years. It is uncommon for primary dysmenorrheato occur
during the first three to six menstrual cycles, when regular ovulation is not
yet well-established. The incidence of primary dysmenorrhea is greatest in
women in their late teens to early 20s and declines with age. Secondary
dysmen-orrhea becomes more common as a woman ages, because it accompanies the
rising prevalence of causal factors. Childbearing does not affect the
occurrence of either pri-mary or secondary dysmenorrhea.
Etiology
Primary dysmenorrhea is caused by
excess prostaglandinF2produced
in the endometrium. Prostaglandin produc-tion in the uterus normally increases
under the influence of progesterone, reaching a peak at, or soon after, the
start of menstruation. With the onset of menstruation, formed prostaglandins
are released from the shedding endome-trium. Prostaglandins are potent
smooth-muscle stimu-lants that cause intense uterine contractions, resulting in
intrauterine pressures that can exceed 400 mm Hg and baseline intrauterine
pressures in excess of 80 mm Hg. Prostaglandin F2α also causes contractions in
smooth mus-cle elsewhere in the body, resulting in nausea, vomiting, and
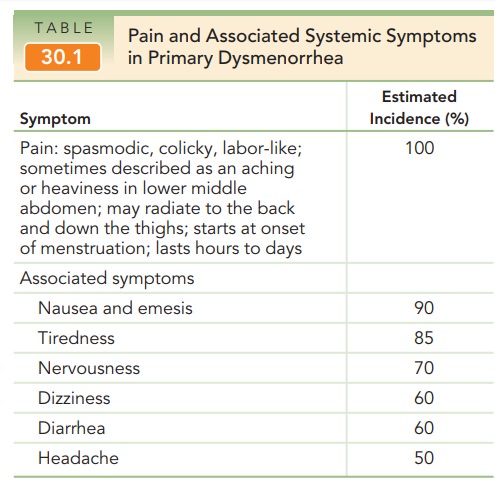
diarrhea (Table 30.1). In addition to the increase in prostaglandins from
endometrial shedding, necrosis of endometrial cells provides increased substrate
arachi-donic acid from cell walls for prostaglandin synthesis. Besides
prostaglandin F2α,
prostaglandin E2 is also pro-duced in the uterus. Prostaglandin E2,
a potent vasodilator and inhibitor of platelet aggregation, has been implicated
as a cause of primary menorrhagia.
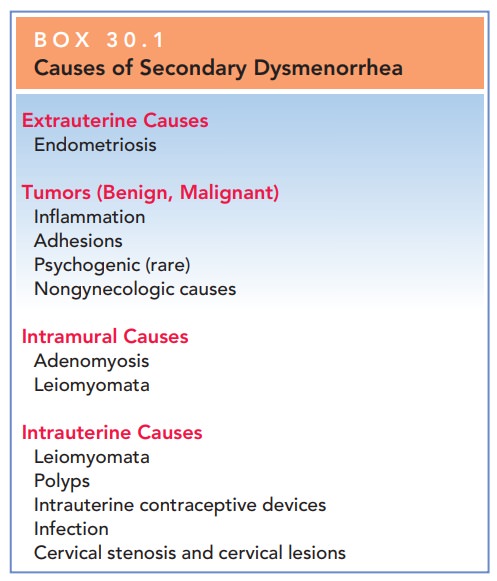
Secondary dysmenorrhea is caused
by structural ab-normalities or disease processes that occur outside the
uterus, within the uterine wall, or within the uterine cavity (Box 30.1).
Common causes of secondary dysmen-orrhea include endometriosis (the presence of ectopic endometrial tissue outside
of the uterus), adenomyosis (the
presence of ectopic endometrial tissue within the myometrium), adhesions, pelvic inflammatory disease, and leiomyomata (uterine fibroids).
Box 30.1
Causes of Secondary Dysmenorrhea
Extrauterine Causes
Endometriosis
Tumors (Benign, Malignant)
Inflammation
Adhesions
Psychogenic (rare)
Nongynecologic causes
Intramural Causes
Adenomyosis
Leiomyomata
Intrauterine Causes
Leiomyomata
Polyps
Intrauterine contraceptive devices
Infection
Cervical stenosis and cervical lesions
SYMPTOMS
In patients with primary
dysmenorrhea, the pain is often diffusely located in the lower abdomen and
suprapubic area, with radiation around or through to the back. The pain is
described as “coming and going” or similar to labor. The patient often
illustrates her description with a fist opening and closing. This pain is
frequently accompanied by moderate to severe nausea, vomiting, and/or diarrhea.
Fatigue, low backache, and headache are also common. Patients often assume a
fetal position in an effort to gain re-lief, and many report having used a
heating pad or hot water bottle in an effort to decrease their discomfort.
In patients with secondary
dysmenorrhea, the pain often lasts longer than the menstrual period. It may
start before menstrual bleeding begins, become worse dur-ing menstruation, then
persist after menstruation ends. Secondary dysmenorrhea often starts later in
life than primary dysmenorrhea.
HISTORY
The specific complaints that an
individual patient has are determined by the underlying abnormality. Therefore,
a careful medical history often suggests the underlying prob-lem and helps
direct further evaluations. Complaints of heavy menstrual flow, combined with
pain, suggest uterine changes such as adenomyosis, leiomyomata, or polyps.
Pelvic heaviness or a change in abdominal contour should raise the possibility
of large leiomyomata or intraabdomi-nal neoplasia. Fever, chills, and malaise
suggest infection. A coexisting complaint of infertility may suggest
endo-metriosis or chronic pelvic inflammatory disease.
ASSESSMENT
For
patients with dysmenorrhea, the physical examination is directed toward
uncovering possible causes of secondary dysmen-orrhea. A pelvic
examination may reveal asymmetry orirregular enlargement of the uterus,
suggesting leiomy-omata or other tumors. Uterine leiomyomata are easily
rec-ognizable on bimanual exam by their smooth contour and rubbery solid
consistency. Adenomyosis may cause a ten-der, symmetrically enlarged, “boggy”
uterus. This diagno-sis is supported by exclusion of other causes of secondary
dysmenorrhea, but definitive diagnosis can be made only by histologic
examination of a hysterectomy specimen. Painful nodules in the posterior
cul-de-sac and restricted motion of the uterus should suggest endometriosis.
Restricted motion of the uterus is also found in cases of pelvic scarring from
adhesions or inflam-mation. Thickening and tenderness of the adnexal
struc-tures caused by inflammation may suggest this diagnosis as the cause of
secondary dysmenorrhea. Cultures of the cervix for Neisseria gonorrhoeae and Chlamydia
trachoma-tis should be obtained if infection is suspected. In some
patients, a final diagnosis may not be established without invasive procedures,
such as laparoscopy.
In evaluating the patient thought
to have primary dysmenorrhea, the most important differential diagnosis is that
of secondary dysmenorrhea. Although the patient’s history is often
characteristic, primary dysmenorrhea should not be diagnosed without a thorough
evaluation to eliminate other possible causes.
Physical
finding of patients with primary dysmenorrhea should be normal.
There should be no palpable abnormalities of the uterus or adnexa, and no abnormalities should be found on speculum or abdominal examinations. Patients exam-ined while experiencing symptoms often appear pale and “shocky,” but the abdomen is soft and nontender, and the uterus is normal.
Therapy
Primary
dysmenorrhea is an appropriate diagnosis for patients with dysmenorrhea in whom
no other clinically identifiable cause is apparent. Patients
with primary dysmenorrhea generallyexperience exceptional pain relief through
the use of non-steroidal
anti-inflammatory drugs (NSAIDs), which areprostaglandin-synthetase
inhibitors. Ibuprofen, naproxen, and mefenamic acid are commonly prescribed
NSAIDs for primary dysmenorrhea. For a time, cyclo-oxygenase inhibitors (COX-2
inhibitors) were becoming the NSAID of choice because of their targeted action.
However, these drugs are now rarely used because of their potential
associ-ation with life-threatening cardiovascular and gastrointesti-nal
effects. Recent studies suggest that continuous low-level topical heat therapy
can provide pain relief comparable to that offered by NSAID therapy without the
systemic side effects that may occur with these drugs.
Therapy with nonsteroidal
anti-inflammatory agents is generally so successful that, if some response is
not evident, the diagnosis of primary dysmenorrhea should be reevaluated. Other
useful components of therapy for pri-mary dysmenorrhea include the application
of heat; exercise; psychotherapy and reassurance; and, on occasion, endocrine
therapy, i.e., oral contraceptives to induce anovulation.
In the rare patient who does not
respond to medical and other therapy and whose pain is so severe as to be
incapacitating, presacral neurectomy
may be a consider-ation. The procedure involves surgical disruption of the
“presacral nerves,” the superior hypogastric plexus, which is found in the
retroperitoneal tissue from the fourth lum-bar vertebra to the hollow over the
sacrum. The risk of intraoperative complications, including injury to adjacent
vascular structures and long-term sequelae such as chronic constipation, limit
the use of this surgical procedure.
For secondary dysmenorrhea, when
a specific diagnosis is possible, therapy directed at the underlying condition
is most likely to succeed. When definitive therapy cannot be used—for example,
in the case of a patient with adenomyosis who wishes to preserve
fertility—symptomatic therapy in the form of analgesics or modification of the
menstrual cycle may be effective.
Combined
oral contraceptives can be useful inpatients who do
not desire childbearing and who do not have contraindications to their use.
They work by suppress-ing ovulation and stabilizing estrogen and progesterone
lev-els, with a resultant decrease in endometrial prostaglandins and
spontaneous uterine activity. Oral contraceptives may be taken in the
traditional 28-day cycle, or in an extended fashion that increases the interval
between menses.
Related Topics