Chapter: Physics : Conducting Materials
Conducting Materials
Conducting Materials
1 Introduction
1.1 Classical free electron theory
1.2 Quantum free electron theory
1.3 Zone
theory (or) Band theory
2 Assumptions (postulates) of Classical free electron theory
3 Basic terms involved in the free electron
theory
4 Success or uses of Classical free electron
theory
5 Drawbacks of Classical free electron theory
6 Expression for Electrical Conductivity
6.1 Expression for electrical conductivity
6.2 Correct expression for electrical
conductivity of conductors
7 Thermal conductivity (K)
7.1 Expression for thermal conductivity (K) of an
electron
8 Wiedemann-franz Law
8.1 Derivation
8.2 By Quantum theory
9 Quantum Free Electron Theory
9.1 Assumptions (Postulates) of Quantum free
electron theory
9.2 Advantages of Quantum free electron theory
9.3 Drawbacks of Quantum free electron theory
10 Fermi – Dirac Distribution Function
10.1 Effect of Temperature on Fermi Function
10.2 Fermi level, Fermi Energy and their
importance
11 Density of States
11.1 Carrier concentration in metals
11.2 Average energy of an electron at 0K
12 Work Function
12.1 Explanation
1
INTRODUCTION
The electron theory of solids explains the structures and
properties of solids through their electronic structure. This theory is
applicable to all solids both metals and non metals. This theory also explains
the bending in solids behavior of conductors and insulators, electrical and
thermal conductivities of solids, elasticity and repulsive forces in solids
etc,.. The theory has been developed in three main stages.
1.1
Classical free electron theory
This theory was developed by Drude and Lorentz. According to this
theory, a metal consists of electrons which are free to move about in the
crystal molecules of a gas it contains mutual repulsion between electrons is
ignored and hence potential energy is taken as zero. Therefore the total energy
of the electron is equal to its kinetic energy.
1.2
Quantum free electron theory
Classical
free electron theory could not explain many physical properties. In classical
free electron theory, we use Maxwell-Boltzman statics which permits all free
electrons to gain energy. In Somerfield developed a new theory, in which he
retained some of the features of classical free electron theory included
quantum mechanical concepts and Fermi-Dirac statistics to the free electrons in
the metals. This theory is called quantum free electron theory. Quantum free
electron theory permits only a few electrons to gain energy.
1.3 Zone
theory (or) Band theory
Bloch developed the theory in which the electrons move in a
periodic field provided by the Lattice concept of holes, origin of Band gap and
effective mass of electrons are the special features of this theory of solids.
This theory also explains the mechanism of super conductivity based on band
theory.
ASSUMPTIONS (POSTULATES) OF CLASSICAL FREE
ELECTRON THEORY
A Solid metal has nucleus
with revolving electrons. The electrons move freely like molecules in a gas.
The free electrons move in a
uniform potential field due to the ions fixed in the lattice.
In the absence of electric
field (E=0), the free electrons move in random directions and collide with each
other. During this collision no loss of energy is observes since the collisions
are elastic as shown in figure.
When the presence of electric
field ( E 0 ) the free electrons are accelerated in the direction
opposite to the direction of applied electric field, as shown in figure.
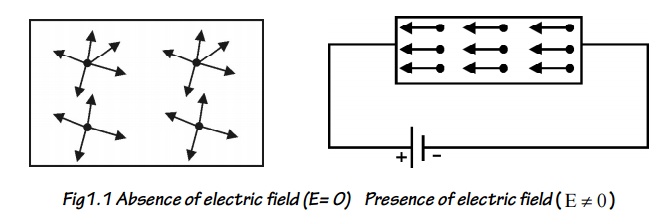
Fig1.1
Absence of electric field (E= 0)
Presence of electric field ( E 0 )
Since the electrons are
assumed to be perfect gas, they obey the laws of classical theory of gases.
Classical free electrons in
the metal obey Maxwell-Boltzmann statistics.
3 BASIC TERMS INVOLVED IN THE FREE ELECTRON THEORY
1. Drift Velocity (Vd)
The drift velocity is defined as the average velocity acquired by
the free electron in particular direction, due to the applied electric field.

2. Mobility
The mobility is defined as the drift velocity (Vd) acquired by the electron per unit
electric field (E).

3. Mean free path
The average distance travelled by a electron between two successive
collision is called mean free path.
4. Mean collision time (or) Collision time
It is
the time taken by the free electron between two successive collision.
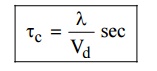
5. Relaxation time
It is
the time taken by the electron to reach equilibrium position from disturbed
position in the presence of electric field.
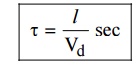
Where l is the distance travelled by the
electron. The value of relaxation time is of the order of 10–14 sec.
6. Band gap (Eg)
Band gap
is the energy difference between the minimum energy of conduction band and the
maximum energy of valence band.
7. Current density (J)
It is
defined as the current per unit area of cross section of an imaginary plane
holded normal to the direction of the flow of current in a current carrying
conductor.
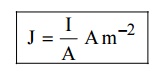
4 SUCCESS OR USES OF CLASSICAL FREE ELECTRON
THEORY
It is used to verify Ohm’s
law.
It is used to explain electrical conductivity ( σ) and thermal conductivity of (K) of metals.
It is used to derive
Widemann-Franz law.
It is used to explain the
optical properties of metal.
5 DRAWBACKS OF CLASSICAL FREE ELECTRON THEORY
It is a macroscopic theory.
According to classical free
electron theory, all the free electrons will absorb energy, but the quantum
free electron theory states that only few electrons will absorb energy.
This theory cannot explain
the Compton effect, Photo-electric effect, para-magnetism and ferromagnetism,
etc.,
This theory cannot explain the
electrical conductivity of semiconductors and insulators.
Dual nature of light
radiation cannot be explained.
The theoretical and
experimental values of specific heat and electronic specific heat are not
matched.

The Lorentz number obtained
by classical theory does not have good agreement with experimental value and
theoritical value, it is rectified by quantum theory.
6
EXPRESSION FOR ELECTRICAL CONDUCTIVITY
Definition
The
electrical conductivity is defined as the quantity of electricity flowing per
unit area per unit time at a constant potential gradient.
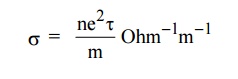
6.1
Expression for electrical conductivity
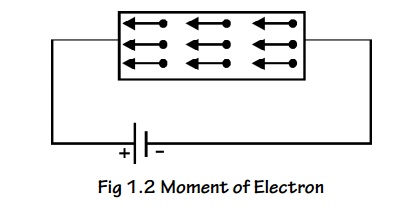
When an electric field (E) is applied to a conductor the free
electrons are accelerated and give rise to current (I) which flows in the
direction of electric filed flows of charges is given in terms of current
density.
Let ‘n’ be the number of electrons per unit volume and ‘e’ be the
charge of the electrons.
The current flowing through a conductor per unit area in unit time
(current density) is given by

The negative sign indicates that the direction of current is in
opposite direction to the movement of electron.
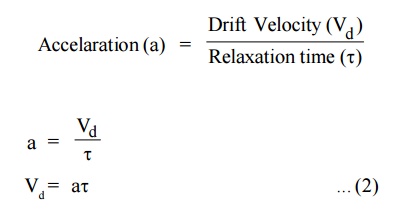
Due to the applied electric field, the electrons acquire an
acceleration ‘a’ can be given by
When an electric field of strength (E) is applied to the conductor,
the force experienced by the free electrons in given by

From Newton’s second Law of motion, the force acquired by the
electrons can be written as
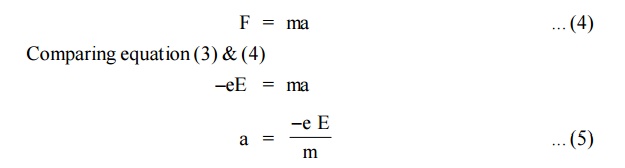
Now, substituting the value of ‘a’ from the equation (2),we get
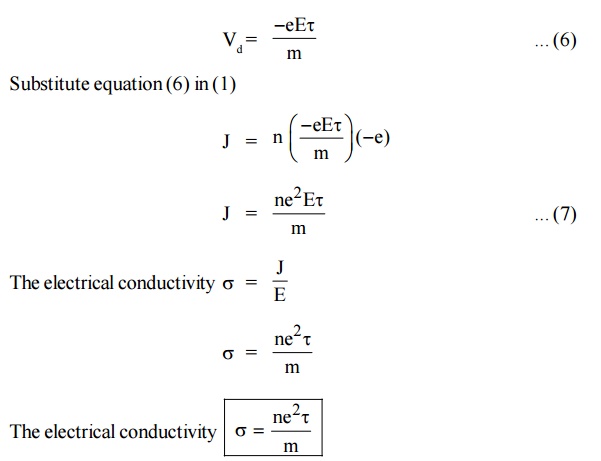
The electrical conductivity of a material is directly proportional
to the free electron concentration in the material.
6.2 Correct expression for electrical conductivity
of conductors
By using the classical free electron theory, quantum free electron
theory and band theory of solids we can get,

Where m*- effective mass of
free electron
Electrical conductivity
Relaxation time
Number of electrons
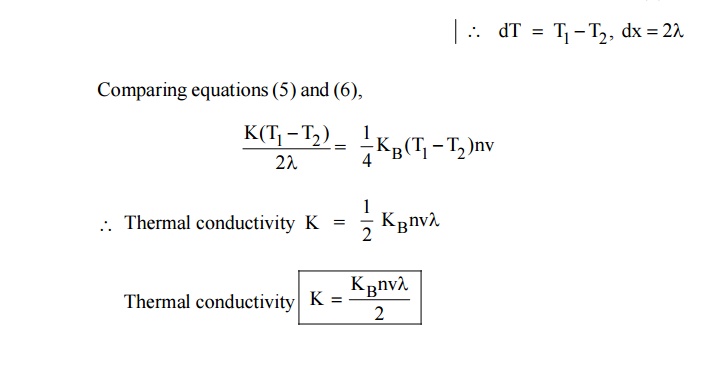
7 THERMAL CONDUCTIVITY (K)
Definition
The
thermal conductivity is defined as the amount of heat flowing through an unit
area per unit temperature gradient.
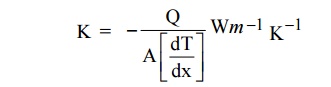
The negative sign indicates that heat flows hot
end to cold end.
Where K is the thermal conductivity of metal. Q is the amount of
heat energy.

In general, the thermal conductivity of a material is due to the
presence of lattice vibrations (ie., photons and electrons). Hence the total
thermal conduction can be written as.

7.1
Expression for thermal conductivity (K) of an electron
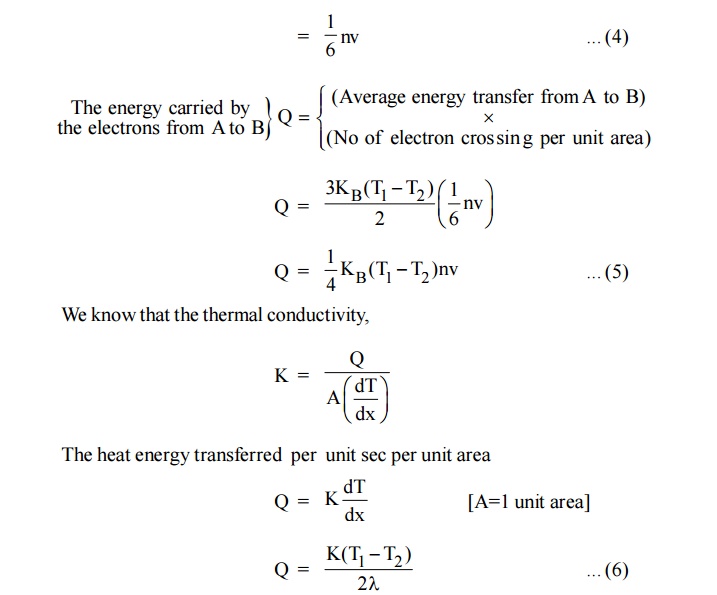
Consider a metal bar with two planes A and B separated by a
distance ‘ ’ from C. Here T1 is hot end and T2 is cold
end. ie., T1 > T2
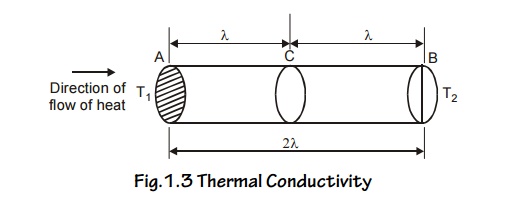
Fig.1.3 Thermal Conductivity
Let ‘n’ be the number of conduction electrons and ‘v’ be the
velocity of the electrons. KB is the Boltzmann constant
From kinetic theory of gases
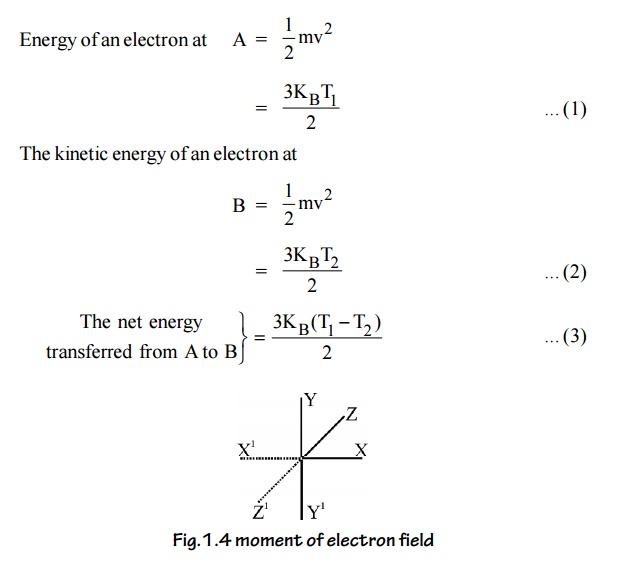
Fig.1.4 moment of electron
field
Let as assume that there is equal probability for the electrons to
move in all the six directions. Each electrons travels with thermal velocity
‘V’ and ‘n’ is the free electron density then on average of 1/6 nv electron
will travel in any one direction.
No. of electrons crossing per unit area in unit time at C
8
WIEDEMANN-FRANZ LAW
Statement
The
ratio between the thermal conductivity (K) and electrical conductivity (σ) of a
metal is directly proportional to the absolute temperature of the metal.
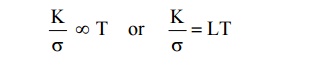
Where L is called Lorentz number, the value of L is 2.44 × 10–8
W K–2 (as per Quantum Mechanical value).
8.1
Derivation
By Classical theory, we can drive Widemann-Franz law using the
expressions for electrical and thermal conductivity of metals.
The expression for thermal conductivity
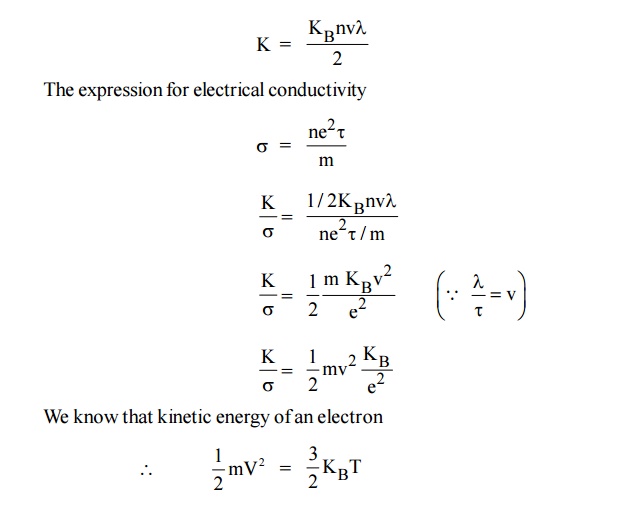
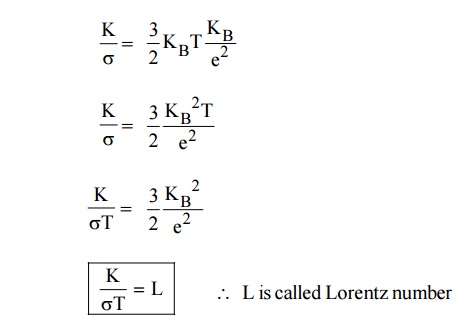
Thus, it is proved that the ratio of thermal conductivity and
electrical conductivity of a metal is directly propotional to the absolute
temperature of the metal.
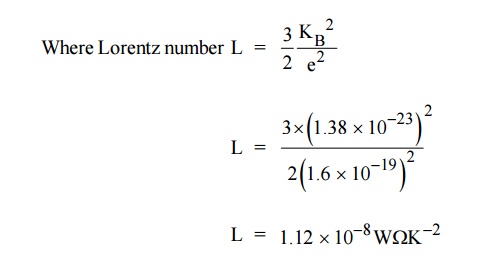
It is found that the classical value of Lorentz number is only one
half of the experimental value (2.44 × 10–8 W K–2). The discrepancy of L value
is the failure of the classical theory (Experimental and
Theoretical). This can be rectified by quantum theory.
8.2 By
Quantum theory
By Quantum theory the mass ‘m’ is replaced by effective mass m*

According to Quantum theory, the expression for thermal
conductivity is modified by considering the electron specific heat as
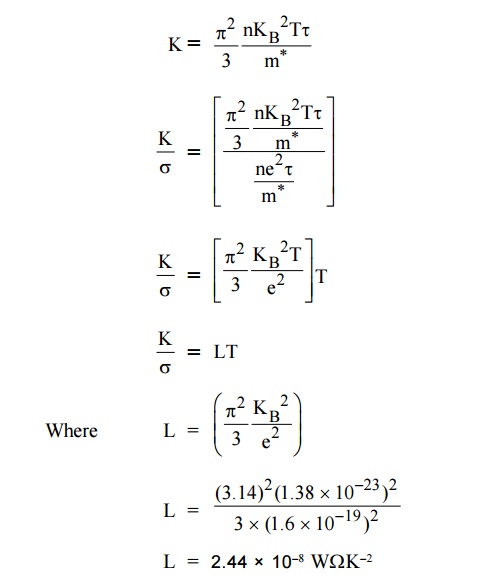
This is gives the correct value of Lorentz number and it in good
agreement with the experiment value.
9 QUANTUM
FREE ELECTRON THEORY
The failure of classical free electron theory paved this way for
Quantum free electron theory. It was introduced by Sommer field in 1928. This
theory is based on making small concepts. This theory was proposed by making
small changes in the classical free electron theory and by retaining most of
the postulates of the classical free electron theory.
9.1 Assumptions (Postulates) of Quantum free
electron theory
In a metal the available free
electrons are fully responsible for electrical conduction.
The electrons move in a
constant potential inside the metal. They cannot come out from the metal
surface have very high potential barrier.
Electrons have wave nature,
the velocity and energy distribution of the electron is given by Fermi-Dirac
distribution function.
The loss of energy due to
interaction of the free electron with the other free electron.
Electron’s distributed in
various energy levels according to Pauli Exclusion Principle.
9.2 Advantages of Quantum free electron theory
This theory explains the
specific heat capacity of materials.
This theory explains photo
electric effect, Compton Effect and block body radiation. etc.
This theory gives the correct
mathematical expression for the thermal conductivity of metals.
9.3 Drawbacks of Quantum free electron theory
This theory fails to
distinguish between metal, semiconductor and Insulator.
It also fails to explain the
positive value of Hall Co-efficient.
According to this theory,
only two electrons are present in the Fermi level and they are responsible for
conduction which is not true.
10 FERMI – DIRAC DISTRIBUTION FUNCTION
Statement
It is an
expression for the distribution of electrons among the energy levels as a
function of temperature, the probability of finding an electron in a particular
energy state of energy E is given by
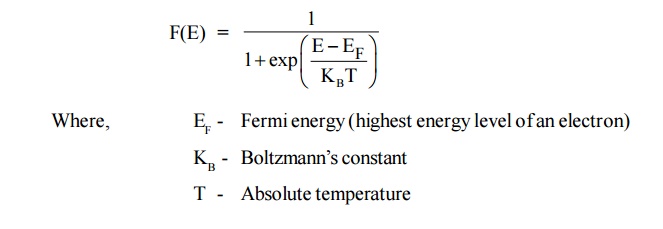
Where, EF - Fermi energy (highest energy level of an
electron)
KB - Boltzmann’s constant
T - Absolute temperature
10.1 Effect of Temperature on Fermi Function
Case 1:
AT = 0 K and E < EF
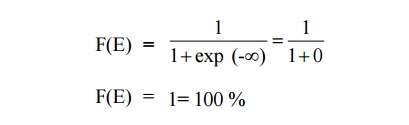
It means that 100% probability for the electrons to occupy the
energy level below the Fermi energy level.
Case 2:
AT = 0 K and E > EF
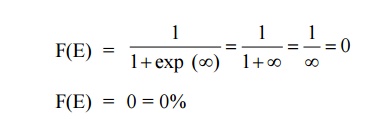
It means that 0% probability (electron) for the electrons to occupy
the energy level above the Fermi energy level.
Case 3 :
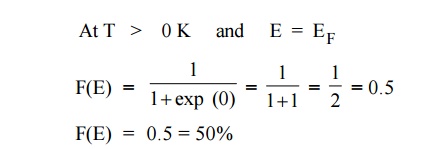
It means that 50% probability for the electrons to occupy the Fermi
energy level. (above Fermi energy level are empty and below Fermi energy level
are filled). At 0 K energy states above EF are empty and below EF
are filled.
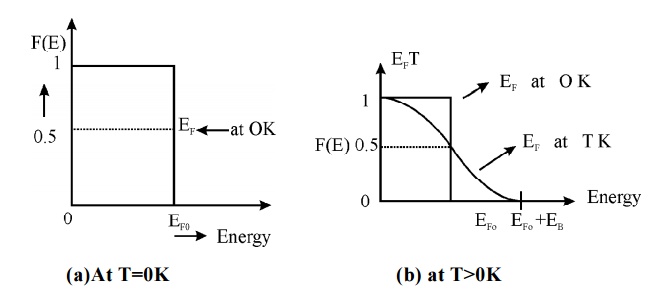
Fig.1.5 Fermi Dirac
distribution function
10.2 Fermi level, Fermi Energy and their
importance
These are defined as the highest energy level filled by the
electrons in that energy level with higher energy values.
Fermi level : The
Fermi level is the highest reference energy level of a particle at absolute
zero.
Importance : It is the reference energy
level which separates the filled energy levels and vacant energy levels.
Fermi energy (EF) : The Fermi energy is the maximum energy of the
quantum state corresponding to Fermi energy level at absolute zero.
Importance : Fermi energy determines the
energy of the particle at any temperature.
11 DENSITY OF STATES
A parameter of interest in the study of conductivity of metals and
semiconductors is the density of states.
The Fermi function F(E) gives only the probability of filling up of
electrons in a given energy state. It does not give the information about the
number of electrons that can be filled in a given energy state, to know that we
should know the number of available energy states called density of states.
Density
of states is defined the as the number of energy states per unit volume in an
energy interval of a metal. It is use to calculate the number of charge
carriers per unit volume of any solid.
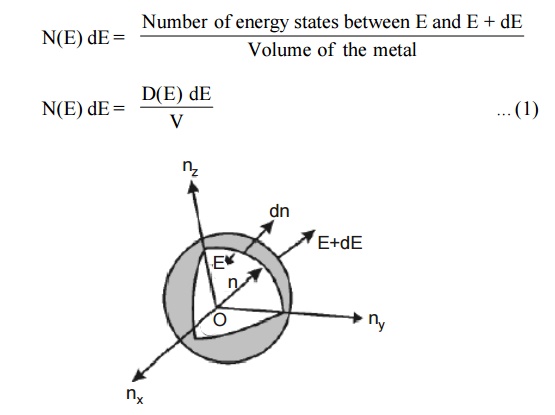
Fig.1.6 Positive octant of n
space
Let us constant a sphere of radius “n” in space with quantum
numbers nn , ny and nz
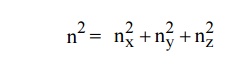
The sphere is further divided into many shells represents a
particular combination of quantum numbers and represents particular energy
value.
Therefore, the number of energy states within a sphere of radius
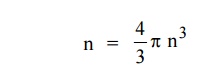
Let us consider two energy values E and E + dE can be found by
finding the number of energy states between the shells of radius n and n+ dn
from the origin. Since the quantum numbers are positive integers, n values can
be defined only in the positive octant of the n – space.
The number of available energy states within the sphere of radius
“n” due to one octant.
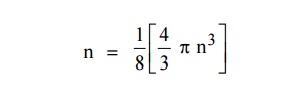
Similarly the number of available energy states within the sphere
of radius n+dn corresponding energy.
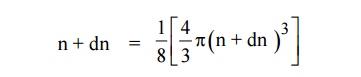
The number of available energy states between the shells of radius
n and n + dn (or) between the energy levels E and E + dE
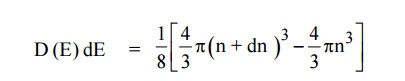
The number of available energy states between the energy interval
dE
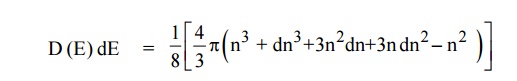
Since the higher powers of dn is very small, dn2 and dn3
terms can be neglected.
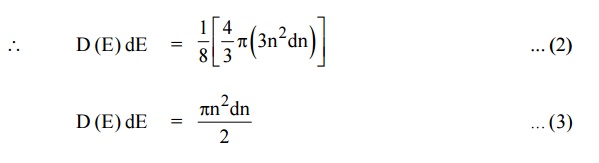
We know that the allowed energy values is

Differentiating equation (4) with respect to ‘n’
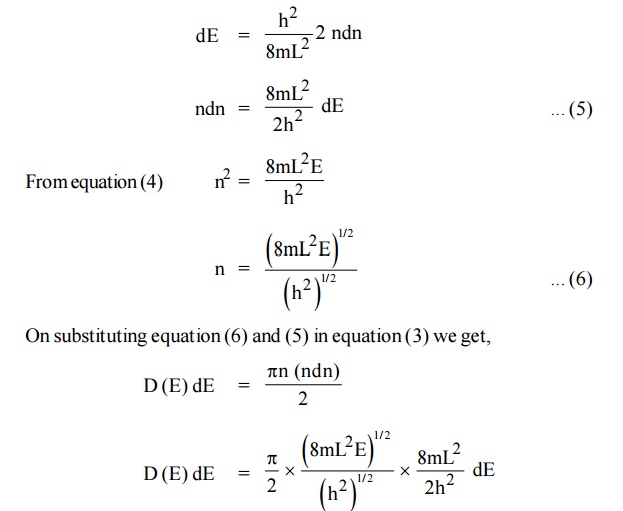
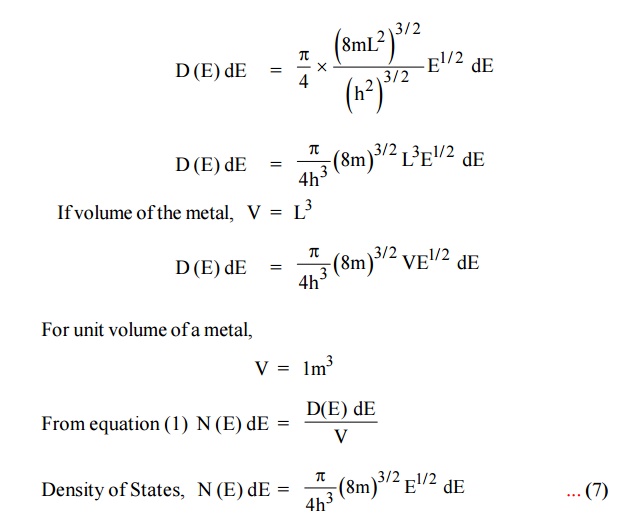
Each electron energy level can accommodate two electrons as per
Pauli’s exclusion principle. (Spin up and Spin down = 2 (e) × density of
states).
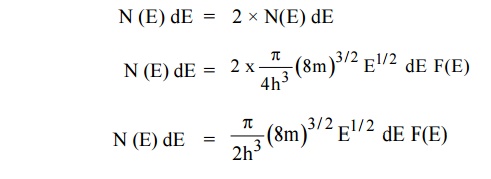
11.1 Carrier concentration in metals
Let N(E) dE represents the number of filled energy states between
the interval of energy dE, normally all the energy states will not be filled
dN = N (E) dE F(E)
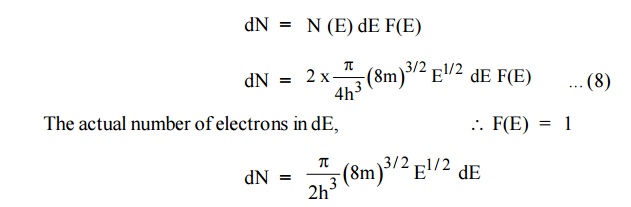
Normally all the states are not filled states, filling of electrons
is a given energy state is given by Fermi-function F(E). Let dn represents the
number of filled energy states.
In this case of material of absolute zero the upper occupied level
is EF and for all the levels below EF, F(E)=1 (at T = 0 K
the maximum energy level that can be occupied by the electron is called Fermi
energy level EF T = 0 K F(E) = 1).
Integrating equation (8) within the limits 0 to EF0 us
can get the number of energy states of electron (N)
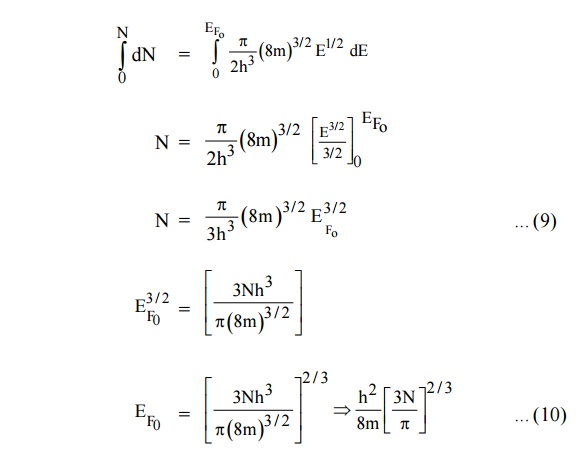
Hence the Fermi energy of a metal depends only on the density of
electrons of that metal.
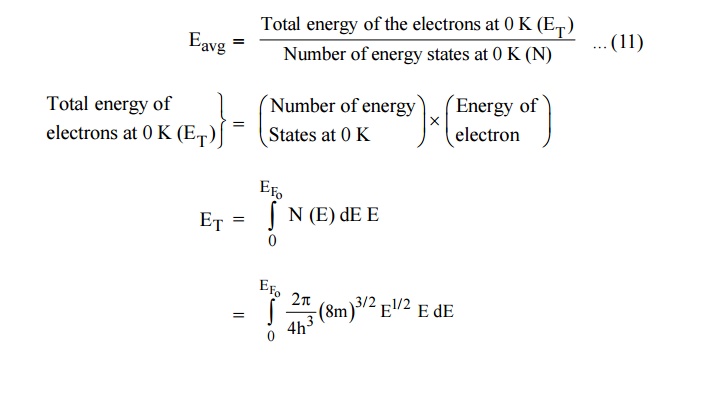
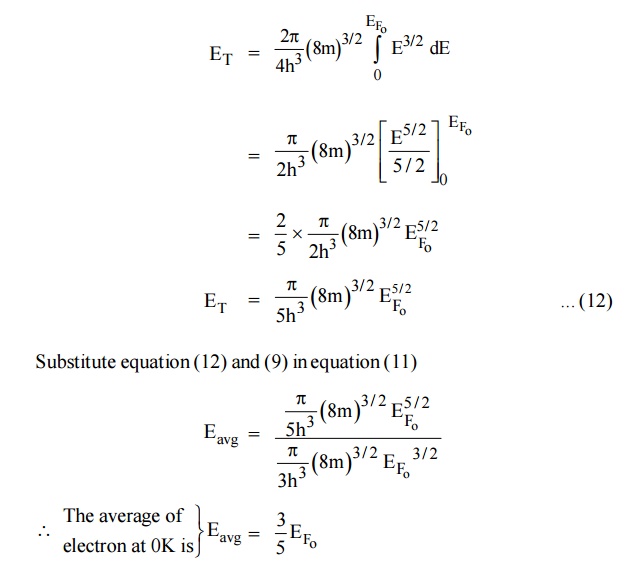
11.2 Average energy of an electron at 0 K
Average energy of electron
12 WORK
FUNCTION
It is
defined as the minimum energy required to remove an electron from the metal
surface at absolute zero (0 K) is called as work function.
12.1 Explanation
Let EF be the maximum energy of an electron called Fermi
energy and EB be the energy of the metal barrier surface.
If we
supply energy EB greater than EF, then no of electron
escapes from the metal.
In order
to make it to escape, an additional amount of energy equal to (EB –
EF ) is required. i.e., EB + (EB – EF
)
This difference in energy EW = EB
– EF is called Work function of a metal.
This
Concept is shown in energy diagram.
Different
metals have different work functions.
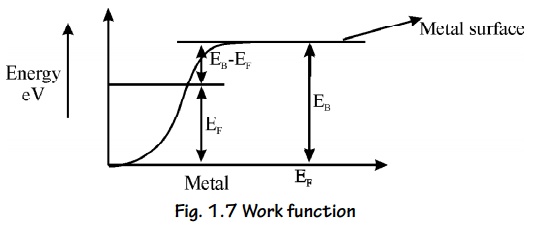
Fig. 1.7 Work function
Work functions for Some Metals
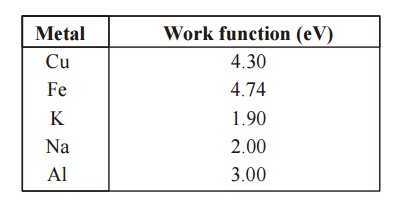
Metal :
Work function (eV)
Cu 4.30
Fe 4.74
K 1.90
Na 2.00
Al 3.00
Related Topics