Chapter: Mechanical : Gas Dynamics and Jet Propulsion : Basic Concepts and Isentropic Flows
Compressible Flows
Compressible Flows
–
Compressible flow - Density
changes
We know that fluids, such as gas, are classified as
Incompressible and Compressible fluids. Incompressible fluids do not undergo
significant changes in density as they flow. In general, liquids are
incompressible; water being an excellent example. In contrast compressible
fluids do undergo density changes. Gases are generally compressible; air being
the most common compressible fluid we can find. Compressibility of gases leads
to many interesting features such
as shocks, which are absent for
incompressible fluids. Gas dynamics is the discipline that studies the flow of
compressible fluids and forms an important branch of Fluid Mechanics.
Compressible vs. Incompressible Flow
o
A flow is classified as incompressible
if the density remains nearly constant.
o
Liquid flows are typically
incompressible.
o
Gas flows are often compressible,
especially for high speeds.
o
Mach number, Ma = V/c is a good
indicator of whether or not compressibility effects are important.
·
Ma < 0.3 : Incompressible
·
Ma < 1 : Subsonic
·
Ma = 1 : Sonic
·
Ma > 1 : Supersonic
§ Ma
>> 1 : Hypersonic
Compressibility:
Measure of the relative volume change with pressure
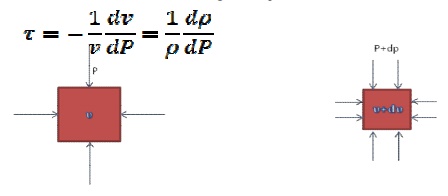
A measure of the relative volume change with
pressure for a given process. Consider a small element of fluid of volume v,
the pressure exerted on the sides of the element is p. Assume the pressure is
now increased by an infinitesimal amount dp. The volume of the element will
change by a corresponding amount dv , here the volume decrease so dv is a
negative quantity. By definition, the compressibility of fluid is
The terms compressibility and incompressibility
describe the ability of molecules in a fluid to be compacted or compressed
(made more dense) and their ability to bounce back to their original density,
in other words, their "springiness." An incompressible fluid cannot
be compressed and has relatively constant density throughout. Liquid is an
incompressible fluid. A gaseous fluid such as air, on the other hand, can be
either compressible or incompressible. Generally, for
theoretical and experimental purposes, gases are
assumed to be incompressible when
they are moving at low speeds--under approximately 220 miles per hour. The
motion of the object traveling through the air at such speed does not affect
the density of the air. This assumption has been useful in aerodynamics when
studying the behavior of air in relation to airfoils and other objects moving
through the air at slower speeds.
In thermodynamics and fluid mechanics,
compressibility is a measure of the relative volume change of a fluid or solid
as a response to a pressure (or mean stress) change.
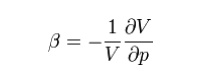
Where V is volume and p is pressure.
The above statement is incomplete, because for any object or system the
magnitude of the compressibility depends strongly on whether the process is
adiabatic or isothermal. Accordingly we define the isothermal compressibility
as:
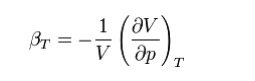
Where the subscript T indicates that the
partial differential is to be taken at constant temperature. The adiabatic
compressibility as:
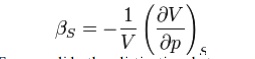
Where S is entropy. For a solid, the
distinction between the two is usually negligible. The inverse of the compressibility
is called the bulk modulus, often denoted K(sometimes B).
Compressibility and
Incompressibility
The terms compressibility and incompressibility
describe the ability of molecules in a fluid to be compacted or compressed
(made more dense) and their ability to bounce back to their original density,
in other words, their "springiness." An incompressible fluid cannot
be compressed and has relatively constant density throughout. Liquid is an
incompressible fluid. A gaseous fluid such as air, on the other hand, can be
either
compressible
or incompressible. Generally, for theoretical
and experimental purposes, gases are
assumed to be incompressible when they are moving at low
speeds--under approximately 220 miles per hour. The motion of the object
traveling through the air at such speed does not affect the density of the air.
This assumption has been useful in aerodynamics when studying the behavior of
air in relation to airfoils and other objects moving through the air at slower
speeds.
However, when aircraft began traveling faster than
220 miles per hour, assumptions regarding the air through which they flew that
were true at slower speeds were no longer valid. At high speeds some of the
energy of the quickly moving aircraft goes into compressing the fluid (the air)
and changing its density. The air at higher altitudes where these aircraft fly
also has lower density than air nearer to the Earth's surface. The airflow is
now compressible, and aerodynamic theories have had to reflect this. Aerodynamic
theories relating to compressible airflow characteristics and behavior are
considerably more complex than theories relating to incompressible airflow. The
noted aerodynamicist of the early 20th century, Ludwig Prandtl, contributed the
Prandtl-Glaubert rule for subsonic airflow to describe the compressibility
effects of air at high speeds. At lower altitudes, air has a higher density and
is considered incompressible for theoretical and experimental purposes.
Compressibility
·
Compressibility of any substance is the
measure of its change in volume under the action of external forces.
·
The normal compressive stress on any
fluid element at rest is known as hydrostatic pressure p and arises as a result
of innumerable molecular collisions in the entire fluid.
·
The degree of compressibility of a
substance is characterized by the bulk modulus of elasticity E
defined as
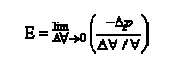
Where Ɐ![]() and
Δpare the changes in the volume and pressure respectively, and Ɐis the initial volume. The negative sign
(-sign) is included to make E positive, since
and
Δpare the changes in the volume and pressure respectively, and Ɐis the initial volume. The negative sign
(-sign) is included to make E positive, since
![]()
Related Topics