Chapter: Pharmaceutical Drug Analysis: Complexometric Analysis
Complexometric Analysis: Theory
THEORY
Complex is a compound that is formed
by the combination of a metal ion with a molecule that is capable of donating electrons, for example :
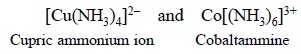
In the above two examples both Cu2+ and Co2+
form complexes with lone pair of electrons present in the neutral molecule
ammonia e.g., N’’H3.
Chelate is a complex that is formed by
the combination of a polyvalent metal ion with a molecule which essentially contains two or more groups that can donate
electrons.
Specifically, disodium ethylenediaminetetraacetate (EDTA)
reacts with polyvalent metal ions to
result in the formation of a fairly stable water-soluble
complex, or a chelate compound.
It is, however, pertinent to mention here that the
predominant state of the dissociated forms of EDTA (viz . Y4–, HY3– , H2Y2–
and H3Y–) is solely dependent upon the pH of the medium
at which complexation takes place :
where, H4Y = ethylenediaminetetraacetic acid, and
Y4– = tetracetate
ion.
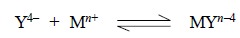
In general, all EDTA
complexation reactions essentially have the ratio of EDTA to metal ion as 1 : 1
Thus, we have :
Ligand is a molecule that affords
groups for attachment to metal ions such as EDTA.
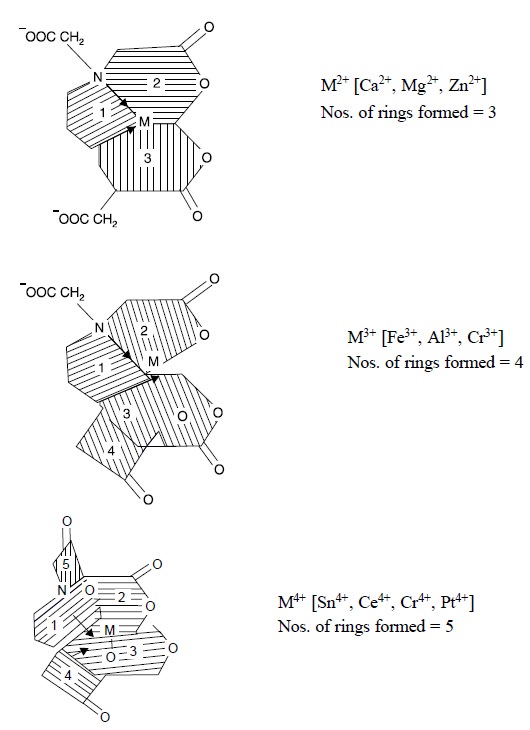
Some examples of polyvalent metal ions are given below :
Bivalent Metal ions : Ca2+, Mg2+,
Zn2+,
Trivalent Metal ions : Fe3+, Al3+,
Cr3+,
Tetravalent Metal ions : Sn4+, Ce4+,
Cr4+, Pt4+.
The structures of the
complexes formed with di-, tri- and tetra-valent metal ions give rise to three, four and five rings respectively as depicted below :
There are various aspects in complex formation and
detection, namely :
(i) Effect of
pH on complexation,
(ii) Stability
of complexes,
(iii)
Colouration of complexes,
(iv)
Titrability of polyvalent metal ions employing disodium edetate, and
(v) Usage of pM
indicators in complexometric titrations.
1. EFFECT OF pH ON COMPLEXATION
Ethylenediamine tetracetic
acid (H4Y) undergoes ionization at four, different stages, namely :
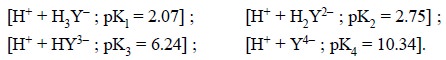
In reality, the actual complexing species is the
tetracetate ion i.e., Y4–
; therefore, complexation will take effect more efficiently and be more stable
in an aikaline medium. Hence, it is evident that EDTA com-plexes of many
divalent metals are quite stable in ammoniacal solution.
As we have seen earlier that the trivalent metal
complexes are normally bound still more firmly due to the formation of four
rings (unlike three rings with divalent metal complexes) and stable in strongly
acidic solutions, for instance : cobalt (Co2+) EDTA complex is
fairly stable in concentrated hydrochloric acid ( −~
11.5 N).
Though a good number of metal-EDTA complexes are found to
be quite stable over a wide-spectrum of pH, yet in actual practice solutions
are normally buffered for two
specific reasons :
(a) to
stabilize the complex formed, and
(b) to achieve
the most distinct colour-change of the indicator.
2. STABILITY OF COMPLEXES
Generally, the formation of a
1 : 1 chelate complex (MX) may be designated by the following equation :

where, M = Metal ion, and
X = Chelating ion.
Hence, the stability constant,
K, may be expressed as :
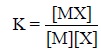
where, items within the ‘square brackets’ represent
activities.
There are two
cardinal factors which influence the stability constant (K), namely :
(a) Elevation
in temperature affords a slight enhancement in the ionization of the complex
and a slight lowering of K, and
(b) Stability
constant is decreased on the addition of electrolytes with no common ion ;
whereas, ethyl alcohol enhances K, perhaps on account of the suppression of
ionization.
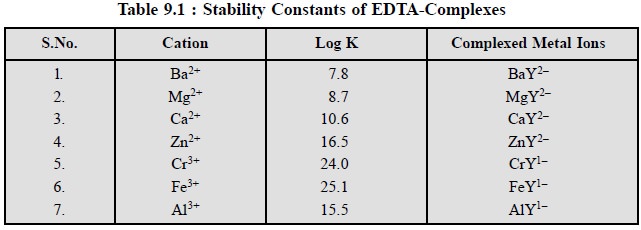
Table 9.1, provides the values
of the logarithms of stability constants (K) of EDTA-complexes of certain
metals normally occurring in pharmaceutical substances :
3. COLOURATION OF COMPLEXES
The formation of EDTA-metal ion complexes invariably
attribute a change in the absorption spectrum pattern which ultimately forms
the basis of a large number of colorimetric assays.
4. TITRABILITY OF POLYVALENT METAL IONS EMPLOYING DISODIUM EDETATE
Ethylenediamine tetracetic acid is found to be sparingly
soluble in water ( −~ 0.2% w/v) whereas its corresponding disodium salt is
almost 50 times more soluble than the parent compound (solubility −~
10% w/v). Therefore, it is the disodium salt of EDTA which is normally used in
complexometric titrations.
In actual practice, whenever the disodium EDTA solution
is added to a solution of a metal ion previously buffered to augment
complexation, it has been observed that initially the rate of change of
concentration of metal ion is rather slow, but interestingly it picks up quite
rapidly as further addition of sodium-EDTA approaches one equivalent.
5. USAGE OF pM INDICATORS IN COMPLEXOMETRIC TITRATIONS
The equivalence point in complexometric titrations is
invariably observed by the help of pM indicators. The relationship amongst pM, concentrations
of ligand, chelate complex and stability constant may be established by the
following equations :
Assuming K as the stability
constant, we have :
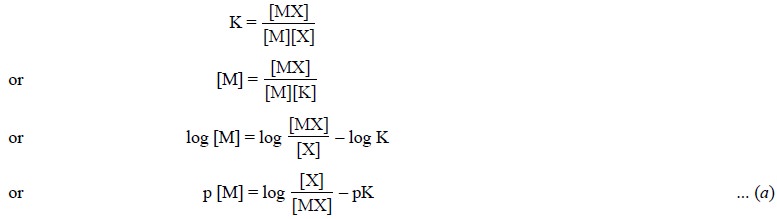
Now, considering Eq. (a),
if a solution is made in such a manner that [X] = [MX], we have :
pM = – pK
or pM = pK′ ... (b)
where, K′ is the dissociation constant.
From Eq. (b) it
may be concluded that a solution having equal activities of free chelating
agent and the metal-complex formed, the concentration of metal ions shall
remain almost constant and would be buffered exactly in a similar fashion as
are H+ ions in a pH-buffer. As we know that the various chelating
agents are mostly basic in character, therefore, the equilibrium attained in a
metal-buffer solution is largely influenced by a change in pH. Hence, it may be
concluded that the amino acid type chelating agents, such as : ethylenediamine
tetracetic acid and ammoniatriacetic acid, when [X] = [MX], pM increases
proportionately with pH until it reaches a value pH 10, thereby attaining a
constant value. Hence, this particular pH is the ‘Ideal pH’ at which complexometric titrations of metals with
chelating agents in buffered solution must be performed.
pM Indicator : It is a dye that serves as a
chelating agent to yield a dye-metal complex, which apparently differs in colour from the original
dye, besides possessing a lower stability constant than the corresponding chelate-metal complex. Hence, the colour
imparted to the solution is mostly attributed due to the dye-complex formed until the end-point, when an equivalent
amount of sodium-EDTA has been incorporated. The critical point at which the
metal-dye complex decomposes to yield free-dye on addition of the slightest
excess of sodium-EDTA, is distinctly shown by a visible change in colour.
Examples :
(i)
Mordant Black 2 : (Syn. : Eriochrome Black T
; Solochrome Black T)
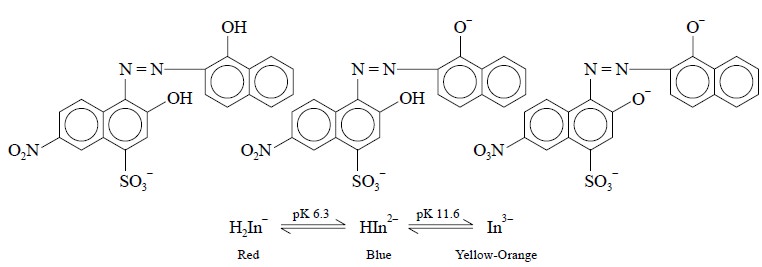
(ii)
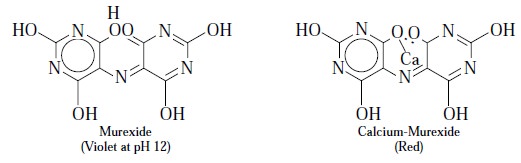
Murexide : (Syn. : Ammonium Purpurate)
Related Topics