Chapter: Basic & Clinical Pharmacology : Dietary Supplements & Herbal Medications
Clinical Aspects of the Use of Botanicals
CLINICAL ASPECTS OF THE USE OF
BOTANICALS
Many United States
consumers have embraced the use of dietary supplements as a “natural” approach
to their health care. Unfortunately, misconceptions regarding safety and
efficacy of the agents are common, and the fact that a substance can be called
“natural” does not of course guarantee its safety. In fact, theseproducts may
be inherently inert, toxic, or may have been adulter-ated, misbranded, or
contaminated either intentionally or unin-tentionally in a variety of ways.
Adverse effects have
been documented for a variety of dietary supplements; however, under-reporting
of adverse effects is likely since consumers do not routinely report, and do
not know how to report an adverse effect if they suspect that the event was
caused by consumption of a supplement. Furthermore, chemical analysis is rarely
performed on the products involved, including those products that are described
in the literature as being linked to an adverse event. This leads to confusion
about whether the primary ingredient or an adulterant caused the adverse
effect. In some cases, the chemical constituents of the herb can clearly lead
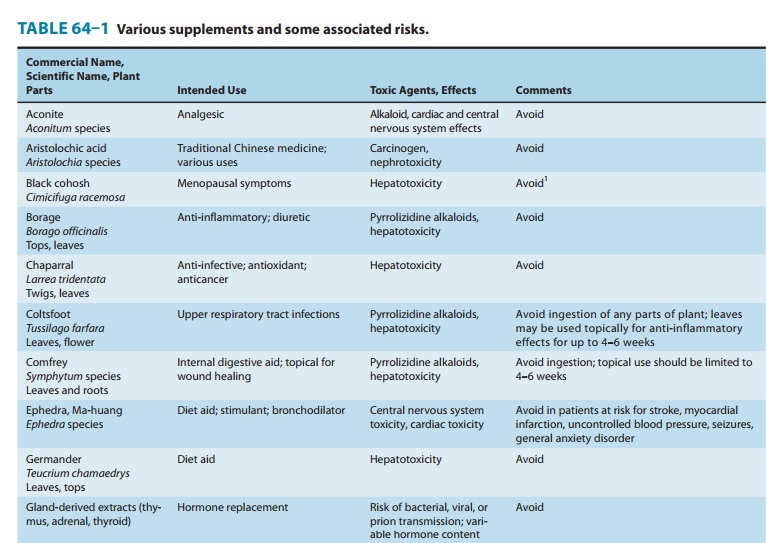
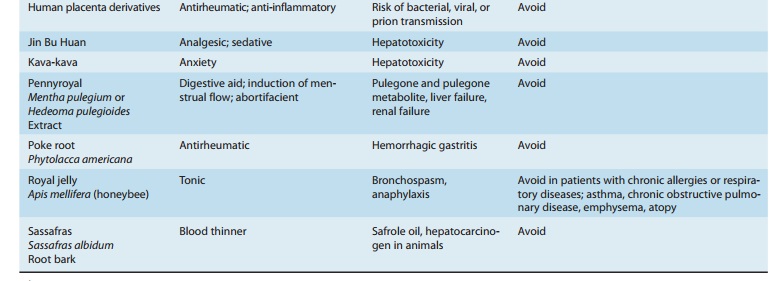
to toxicity. Some of the herbs that should be used cautiously or not at all are
listed in Table 64–1.
An important risk
factor in the use of dietary supplements is the lack of adequate testing for
drug interactions. Since botanicals may contain hundreds of active and inactive
ingredients, it is very difficult and costly to study potential drug
interactions when they are combined with other medications. This may present
signifi-cant risks to patients.
Related Topics