Chapter: Modern Pharmacology with Clinical Applications: β-Lactam Antibiotics
Cephalosporins
CEPHALOSPORINS
The cephalosporins are
semisynthetic antibiotics derived from products of various microorganisms,
including Cephalosporium and Streptomyces. All cephalosporins have a
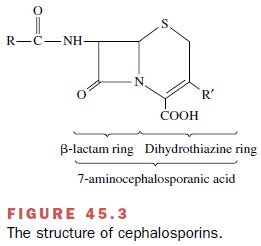
7-aminocephalosporanic acid composed of a di-hydrothiazine ring fused to a β-lactam
ring (Fig. 45.3). As with the penicillins, the cephalosporin β-lactam ring is
the chemical group associated with antibacterial activity. The different
pharmacological, pharmacokinetic, and an-tibacterial properties of individual
cephalosporins result from substitution of various groups on the basic
mole-cule. Cephalosporins also vary in acid stability and β-lactamase
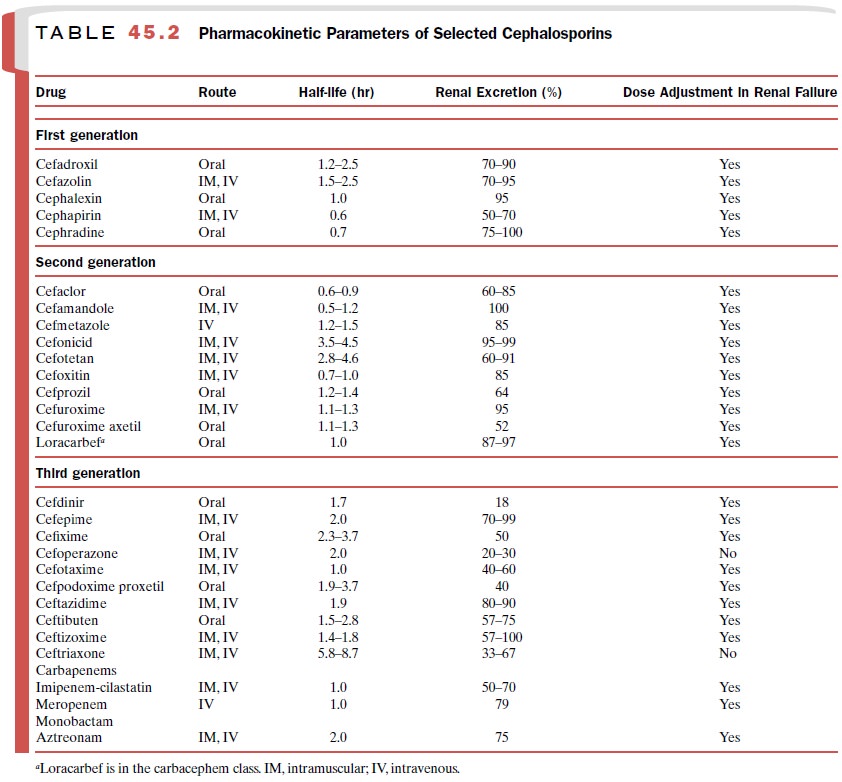
susceptibility. Table 45.2 shows the large num-ber of available cephalosporins.
The β-lactamases
(penicillinases) inactivate some cephalosporins but are much less efficient
than are the cephalosporinases ( β-lactamases specific for the cephalosporins).
Resistance to cephalosporins also re-sults from modification of microbial PBPs.
Antibacterial Spectrum
The cephalosporins are classified into generations (Table 45.2) according to their antibacterial spectrum and stability to β-lactamases. The first-generation cephalosporins have in vitro antimicrobial activity against streptococci, methicillin-sensitive S. aureus, and a few gram-negative bacilli. The second-generation cepha-losporins have greater stability against β-lactamase inac-tivation and possess a broader spectrum of activity to in-clude gram-positive cocci, gram-negative organisms, and anaerobes. Among the second-generation cephalo-sporins, the cephamycins (cefoxitin [Mefoxin], cefotetan [Cefotan], and cefmetazole [Zefazone]) have the most activity against Bacteroides fragilis. The extended-spectrum, or third-generation, cephalosporins possess a high degree of in vitro potency and β-lactamase stability and a broader spectrum of action against many common gram-negative bacteria and anaerobes while retaining good activity against streptococci. Third-generation cephalosporins are less active against staphylococci than the earlier generations. The agents with the greatest ac-tivity against P. aeruginosa are cefepime, cefoperazone, and ceftazidime. Cefepime has been called a fourth-gen-eration cephalosporin because of its great in vitro activ-ity against several gram-positive and gram-negative or-ganisms.
The distinction between third and fourth generation may be irrelevant,
however, since clinical outcomes are similar in human trials comparing
ce-fepime and other third-generation cephalosporins. None of the cephalosporins
adequately treats infections caused by Enterococcus
faecalis, E. faecium, MRSA, or L.
monocytogenes.
Absorption, Distribution, Metabolism, and Excretion
Most parenteral
cephalosporins have good bioavailabil-ity after intramuscular injection, and a
few members of each cephalosporin generation have good oral bioavail-ability
(Table 45.2). The ester prodrugs cefuroxime ax-etil (Ceftin) and cefpodoxime proxetil (Vantin) are oral formulations in which the ester is hydrolyzed
during drug passage through the intestinal mucosa; the free cephalosporin
enters the systemic circulation. Con-comitant ingestion of food reduces the
bioavailability of some cephalosporins, e.g., cefaclor (Ceclor), and there-fore, these compounds should be administered on
an empty stomach.
The cephalosporins distribute
in satisfactory con-centrations to most tissues except the central nervous
system. Only cefepime, cefuroxime (Zinacef),
cefo-taxime (Claforan), ceftriaxone (Rocephin), and cef-tazidime (Fortaz) achieve therapeutic
concentrations in cerebrospinal fluid. Cefotaxime and ceftriaxone are
an-tibiotics of first choice for the empirical treatment of brain abscess and
meningitis.
There is considerable
variation in the protein bind-ing among the cephalosporins. Drugs like
ceftriaxone that have extensive protein binding (85–95%) may dis-place
bilirubin from serum albumin. Consequently, cef-triaxone may increase the risk
of kernicterus in jaun-diced neonates.
Urinary excretion is the
major elimination path for most cephalosporins. When prescribing cephalosporins
to patients with renal failure, practitioners must consider dose reduction or
dose interval extension (Table 45.2). Renal tubular secretion contributes to
the elimination of some cephalosporins, and an increase in cephalosporin plasma
concentrations may occur when probenecid blocks renal tubular secretion of
cephalosporins. Biliary elimination is important for some cephalosporins.
Cefmetazole, cefoperazone (Cefobid),
cefoxitin, and cef-triaxone achieve biliary concentrations greater than those
in plasma. After parenteral administration of cef-operazone, 70% of the dose
appears in the bile within 24 hours. Practitioners should decrease the dose of
cefop-erazone when prescribing for patients with hepatic fail-ure or biliary
obstruction. Metabolism is not a major elimination path for most
cephalosporins. Cefotaxime is one of the few cephalosporins having an active
metabo-lite, desacetyl cefotaxime.
Clinical Uses
The first-generation
cephalosporins have activity against most of the bacterial pathogens that
colonize skin and infect wounds. Consequently, first-generation cephalosporins
are useful in antimicrobial prophylaxis before surgery. Second-generation
cephalosporins are used to treat infections caused by susceptible organ-isms.
For example, cefoxitin and cefotetan have good anaerobic activity, and they
have utility in the treatment and prophylaxis of lower abdominal and
gynecological infection. A broad spectrum of antibacterial activity makes
third-generation cephalosporins important in the treatment of a wide range of
infections, including Lyme disease, pneumonia, peritonitis, and sepsis
syn-drome.
Adverse Effects
The cephalosporins have good
safety profiles. The overall incidence of adverse events attributed to
cephalosporins is between 1 and 10%. The most common adverse drug reactions are
rashes (1–5%), eosinophilia (3–10%), gas-trointestinal symptoms (3%),
hematological abnormali-ties (1–2%), phlebitis (2%), and fever ( 1%).
Ana-phylactic reactions to cephalosporins are rare ( 0.02%).
Because of cross-reactions
between cephalosporins and penicillins, caution should be used when prescribing
cephalosporins to patients with penicillin allergy. If a patient had
anaphylaxis, angioedema, or urticaria fol-
lowing penicillin use,
cephalosporins should be avoided. Among patients with morbilliform rashes
(resembling measles) after penicillin, the majority (95%) will toler-ate
cephalosporins without adverse effects and with no increased risk of
anaphylaxis. When evaluating patients with histories of allergic penicillin
reactions, practition-ers may order penicillin skin tests to screen potential
cephalosporin recipients. The frequency of allergic reac-tions to
cephalosporins is 1.7% in patients with histories of type I penicillin
reactions and negative penicillin skin tests. Most patients with negative
penicillin skin tests may receive cephalosporins safely.
The cephalosporins are
valuable because of their broad spectrum of antimicrobial activity. However,
their bactericidal action alters gut flora and selects for overgrowth of
resistant organisms. Cephalosporins have been associated with superinfections
with Clostridium difficile, enterococci,
MRSA, coagulase-negative staphylococci, P.
aeruginosa, and Candida albi-cans. Overgrowth
by toxigenic C. difficile occasionally causes pseudomembranous colitis in
patients treated with cephalosporins. Some third-generation cepha-losporins
induce production of extended-spectrum β-lactamases (ESBLs) in P. aeruginosa. The ESBLs can transfer to
various Enterobacteriaceae and produce or-ganisms resistant to almost all β-lactam
antibiotics.
Bleeding is an uncommon but
serious side effect of some cephalosporins. The N-methylthiotetrazole (MTT) side chain on the R substituent
inhibits production of active vitamin K. Cephalosporins with the MTT side chain
(cefamandole, cefmetazole, cefoperazone, cefote-tan) are associated with
hypoprothrombinemia, coagu-lation abnormalities, and bleeding. In addition, the
MTT cephalosporins increase the effect of oral antico-agulants. Bleeding or
coagulation abnormalities caused by MTT cephalosporins can be treated or
prevented with supplemental vitamin K. Additional bleeding problems may result
from antiplatelet effects. The MTT side chain confers a structure and activity
similar to that of disulfiram, so patients taking MTT cephalosporins who also
ingest alcohol may develop symptoms similar to the disulfiram reaction.
Children and adults receiving
high doses of ceftriax-one may develop gallbladder sludge (pseudolithiasis).
While most patients with sludge have no symptoms, oc-casionally the sludge
identified by abdominal ultra-sonography has led to laparotomy. Biliary sludge
usu-ally disappears after discontinuation of ceftriaxone.
Related Topics