Chapter: Modern Pharmacology with Clinical Applications: β-Lactam Antibiotics
Carbapenems and Carbacephems
CARBAPENEMS AND
CARBACEPHEMS
The newest classes of β-lactam
antibiotics are the car-bapenems and carbacephems. Their mechanism of action is
the same as those of the other β-lactam antibi-otics.
Imipenem
The first carbapenem,
imipenem–cilastatin (Primaxin), is a chemically stable analogue of thienamycin
pro-duced by Streptomyces cattleya.
The antibacterial spec-trum of imipenem is among the broadest of all of the β-lactam
antibiotics. Imipenem is active against most gram-positive, gram-negative, and
anaerobic bacteria. When compared with the in vitro activities of
third-generation cephalosporins, imipenem is more potent against E. faecalis, B. fragilis, and P. aeruginosa. Imipenem’s stability
against β-lactamases is attributa-ble to the trans position of the
6-hydroxyethyl side chain on the β-lactam ring. Organisms resistant to imipenem
include E. faecium, Stenotrophomonas
mal-tophilia, and MRSA.
Imipenem–cilastatin is only
available for intramus-cular or intravenous administration because oral
bioavailability is poor. The enzyme, dehydropeptidase I, present in renal
tubules, converts imipenem to an inac-tive metabolite. To decrease metabolic
clearance, imipenem is combined with cilastatin, an inhibitor of
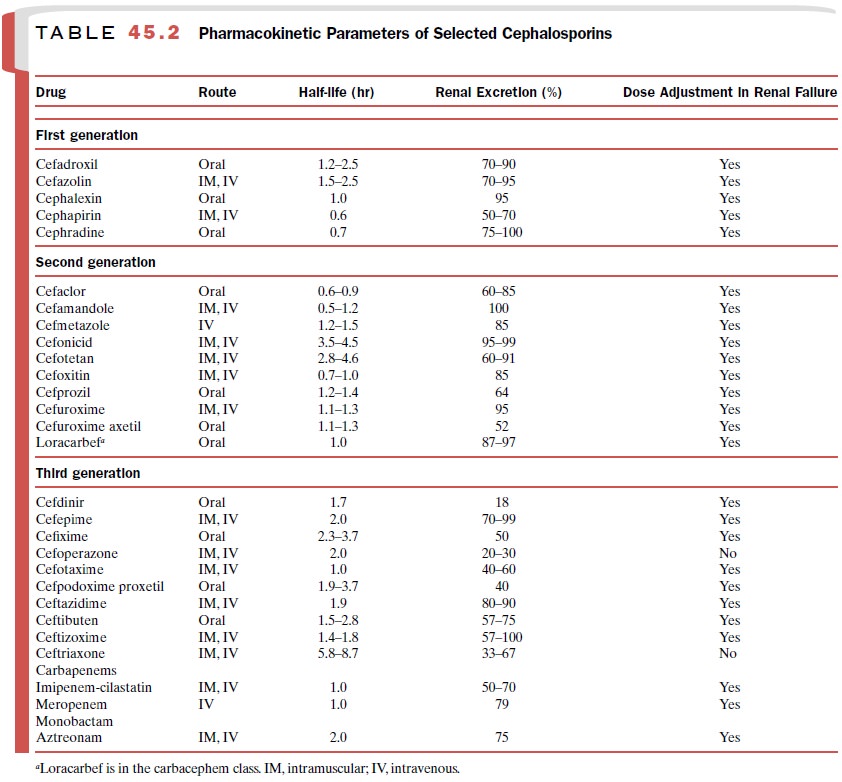
dehydropeptidase I. Additional pharmacokinetic infor-mation appears in Table
45.2.
Imipenem–cilastatin is one of
the drugs of first choice for the empirical therapy of many polymicrobial
pulmonary, intraabdominal, and soft tissue infections. The notable adverse
effect of imipenem–cilastatin is seizures affecting 1% of patients. Risk
factors for seizures are old age, head trauma, previous seizure dis-order,
cerebrovascular accident, and renal failure. Among patients with a history of
penicillin allergy, 10% are cross-sensitive to imipenem–cilastatin.
Meropenem
Meropenem (Merrem) is another carbapenem
antibi-otic with a broad spectrum of activity comparable to that of imipenem. A
methyl group attached at the one-position on the five-member ring confers
stability to de-hydropeptidase I. Consequently, meropenem does not require
administration with cilastatin. When compared in human trials,
imipenem–cilastatin and meropenem achieve similar clinical outcomes in patients
with seri-ous intraabdominal and soft tissue infections. Both
imipenem–cilastatin and meropenem are used to treat infections caused by highly
resistant Klebsiella pneumo-niae producing
ESBLs. The major clinically relevant dis-tinction between imipenem–cilastatin
and meropenem is the lower likelihood of seizures associated with meropenem.
Loracarbef
Loracarbef (Lorabid) is a synthetic β-lactam
antibiotic of the carbacephem class. The chemical structure of lo-racarbef is
similar to that of cefaclor. Selected pharma-cokinetic information appears in
Table 45.2. Lora-carbef’s spectrum of antibacterial activity resembles those of
the second-generation cephalosporins. Com-parative clinical trials reveal
similar outcomes in pa-tients treated with cefaclor, cefprozil, and loracarbef.
Related Topics