Chapter: Basic & Clinical Pharmacology : Special Aspects of Geriatric Pharmacology
Central Nervous System Drugs
CENTRAL NERVOUS SYSTEM DRUGS
Sedative-Hypnotics
The half-lives of many
benzodiazepines and barbiturates increase by 50–150% between ages 30 and 70.
Much of this change occurs during the decade from 60 to 70. For some of the
benzodiaz-epines, both the parent molecule and its metabolites (produced in the
liver) are pharmacologically active . The age-related decline in renal function
and liver disease, if present, both contribute to the reduction in elimination
of these compounds. In addition, an increased volume of distribution has been
reported for some of these drugs. Lorazepam and oxazepam may be less affected
by these changes than the other benzodiazepines. In addi-tion to these pharmacokinetic
factors, it is generally believed that the elderly vary more in their
sensitivity to the sedative-hypnotic drugs on a pharmacodynamic basis as well.
Among the toxicities of these drugs, ataxia and other signs of motor impairment
should be particularly watched for in order to avoid accidents.
Analgesics
The opioid analgesics
show variable changes in pharmacokinetics with age. However, the elderly are
often markedly more sensitive to the respiratory effects of these agents
because of age-related changes in respiratory function. Therefore, this group
of drugs should be used with caution until the sensitivity of the particular
patient has been evaluated, and the patient should then be dosed appropriately
for full effect. Unfortunately, studies show that opi-oids are consistently underutilized in patients who require
strong analgesics for chronic painful conditions such as cancer. There is no
justification for underutilization of these drugs, especially in the care of
the elderly, and good pain management plans are read-ily available (see
Morrison, 2006; Rabow, 2011).
Antipsychotic & Antidepressant Drugs
The traditional
antipsychotic agents (phenothiazines and haloperi-dol) have been very heavily
used (and probably misused) in the management of a variety of psychiatric
diseases in the elderly. There is no doubt that they are useful in the
management of schizophre-nia in old age, and also in the treatment of some
symptoms associ-ated with delirium, dementia, agitation, combativeness, and a
paranoid syndrome that occurs in some geriatric patients . However, they are
not fully satisfactory in these geriatric conditions, and dosage should not be
increased on the assumption that full control is possible. There is no evidence
that these drugs have any beneficial effects in Alzheimer’s dementia, and on
theoretical grounds the antimuscarinic effects of the phe-nothiazines might be
expected to worsen memory impairment and intellectual dysfunction .
Much
of the apparent improvement in agitated and combative patients may simply
reflect the sedative effects of the drugs. When a sedative antipsychotic is
desired, a phenothiazine such as thior-idazine is appropriate. If sedation is
to be avoided, haloperidol or an atypical antipsychotic is more appropriate.
Haloperidol has increased extrapyramidal toxicity, however, and should be
avoided in patients with preexisting extrapyramidal disease. The
phenothi-azines, especially older drugs such as chlorpromazine, often induce
orthostatic hypotension because of their α-adrenoceptor-blocking effects.
They are even more prone to do so in the elderly. Dosage of these drugs should
usually be started at a fraction of that used in young adults.Lithium is often
used in the treatment of mania in the aged. Because it is cleared by the kidneys,
dosages must be adjusted appropriately and blood levels monitored. Concurrent
use of thi-azide diuretics reduces the clearance of lithium and should be
accompanied by further reduction in dosage and more frequent measurement of
lithium blood levels.
Psychiatric
depression is thought to be underdiagnosed and undertreated in the elderly. The
suicide rate in the over-65 age group (twice the national average) supports
this view. Unfortunately, the apathy, flat affect, and social withdrawal of
major depression may be mistaken for senile dementia. Clinical evidence
suggests that the elderly are as responsive to antidepressants (of all types)
as younger patients but are more likely to experience toxic effects. This
factor along with the reduced clearance of some of these drugs underlines the
importance of careful dosing and strict atten-tion to the appearance of toxic
effects. If a tricyclic antidepressant is to be used, a drug with reduced
antimuscarinic effects should be selected, eg, nortriptyline or desipramine
(see Table 30–2). To minimize autonomic effects, a selective serotonin reuptake
inhibi-tor (SSRI) may be chosen.
Drugs Used in Alzheimer’s Disease
Alzheimer’s disease is
characterized by progressive impairment of memory and cognitive functions and
may lead to a completely vegetative state, resulting in massive socioeconomic
disruption, and early death. Prevalence increases with age and may be as high
as 20% in individuals over 85. Both familial and sporadic forms have been
identified. Early onset of Alzheimer’s disease is associ-ated with several gene
defects, including trisomy 21 (chromosome 21), a mutation of the gene for
presenilin-1 on chromosome 14, and an abnormal allele, ε4, for the lipid-associated protein, ApoE, on
chromosome 19. Unlike the normal form, ApoE ε2, the ε4 form facilitates the formation of amyloid β deposits.
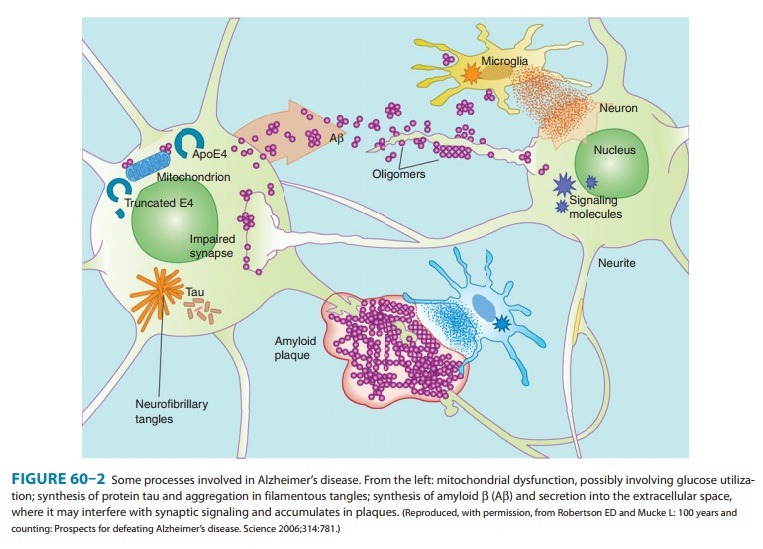
Pathologic changes
include increased deposits of amyloid β peptide in the cerebral cortex, which eventually
forms extracellular plaques and cerebral vascular lesions, and intraneuronal
fibrillary tangles consisting of the tau protein (Figure 60–2). There is a
progressive loss of neurons, especially cholinergic neurons, and thinning of
the cortex. The loss of cholinergic neurons results in a marked decrease in
choline acetyltransferase and other markers of cholinergic activity. Patients
with Alzheimer’s disease are often exquisitely sensitive to the central nervous
system toxicities of drugs with antimuscarinic effects. Some evidence
implicates excess excitation by glutamate as a contributor to neuronal death.
In addition, abnormalities of mitochondrial function may contribute to neuronal
death.
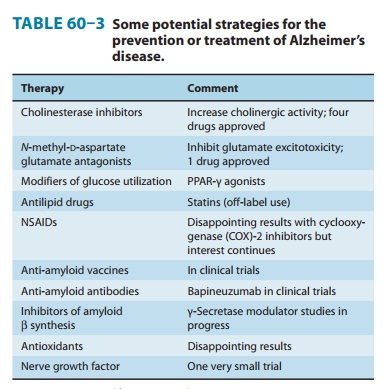
Many methods of
treatment of Alzheimer’s disease have been explored (Table 60–3). Most
attention has been focused on the cholinomimetic drugs because of the evidence
of loss of cholin-ergic neurons. Monoamine oxidase (MAO) type B inhibition with
selegiline (L-deprenyl) has been
suggested to have some beneficial effects. One drug that inhibits N-methyl-D-aspartate (NMDA) glutamate receptors is available , and
“ampakines,” substances that facilitate synaptic activity at glutamate AMPA
receptors, are under intense study. Some evidence suggests that lipid-lowering
statins are beneficial. Rosiglitazone, a PPAR-γ (per-oxisome proliferator-activated
receptor-gamma) agent, has also been reported to have beneficial effects in a
preliminary study. Unfortunately, this drug is associated with increased
cardiovascu-lar risk and its use has been restricted . So-called cerebral
vasodilators are ineffective.
Tacrine (tetrahydroaminoacridine, THA), a long-acting cho-linesterase inhibitor and muscarinic modulator, was the first drug shown to have any benefit in Alzheimer’s disease.
Because of its hepatic toxicity,
tacrine has been almost completely replaced in clinical use by newer
cholinesterase inhibitors: donepezil,
rivastig-mine, and galantamine. These
agents are orally active, have ade-quate penetration into the central nervous
system, and are much less toxic than tacrine. Although evidence for the benefit
of cho-linesterase inhibitors (and memantine; ) is statistically significant,
the clinical benefit from these drugs is modest and temporary.
The cholinesterase
inhibitors cause significant adverse effects, including nausea and vomiting,
and other peripheral cholinomi-metic effects. These drugs should be used with
caution in patients receiving other drugs that inhibit cytochrome P450 enzymes
(eg, ketoconazole, quinidine;).
Excitotoxic
activation of glutamate transmission via NMDA receptors has been postulated to
contribute to the pathophysiol-ogy of Alzheimer’s disease. Memantine binds to NMDA receptor channels in a use-dependent manner
and produces a noncompeti-tive blockade. This drug appears to be better
tolerated and less toxic than the cholinesterase inhibitors. Memantine is
available as Namenda in 5 and 10 mg oral tablets.
Related Topics