Chapter: Clinical Anesthesiology: Perioperative & Critical Care Medicine: Cardiopulmonary Resuscitation
Cardiopulmonary Resuscitation: Defibrillation
DEFIBRILLATION
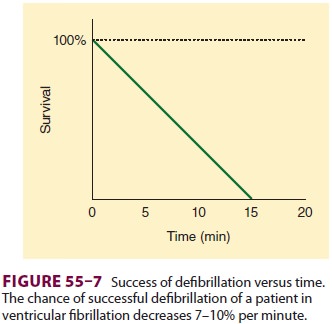
Ventricular fibrillation develops most
commonly in adults who experience nontraumatic cardiac arrest. The time from
collapse to defibrillation is the most important determinant of survival. The
chances for survival decline 7–10% for every minute without defibrillation (
Figure 55–7). Therefore, patients who have cardiac arrest should be
defibrillated at the earliest possible moment. Health care personnel working in
hospitals and ambulatory carefacilities must be able to provide early
defibrillation to collapsed patients with ventricular fibrillation as soon as
possible. Shock should be delivered within 3 min (+-1 min) of arrest.
There is no definite relationship between the energy requirement for
successful defibrillation and body size. A shock with too low an energy
(current) level will not successfully defibrillate; conversely, too high an
energy level may result in functional and morphological injury. Defibrillators
deliver energy in either monophasic or biphasic waveforms. Increasingly,
biphasic waveforms are recommended for cardioversion as they achieve the same
degree of success but with less energy and theoretically less myocardial
damage.
In many institutions, automated external
defi-brillators (AEDs) are available. Such devices are increasingly being used
throughout the community by police, firefighters, security personnel, sports
marshals, ski patrol members, and airline flight attendants, among others. They
are placed in any public location where 20,000 or more people pass by every
day. AEDs are technologically advanced, microprocessor-based devices that are capable
of electrocardiographic analysis with very high specific-ity and sensitivity in
differentiating shockable from nonshockable rhythms. All AEDs manufactured
today deliver some type of biphasic waveform shock. Compared with monophasic
shocks, biphasic shocks deliver energy in two directions with
equivalent effi-cacy at lower energy levels and possibly with less myocardial
injury. These devices deliver impedance-compensating shocks employing either
biphasic truncated exponential (BTE) or rectilinear (RBW) morphology. Biphasic
shocks delivering low energy for defibrillation (120–200 joule [J]) have been
found to be as or more effective than 200–360 J monophasic damped sine (MDS)
waveform shocks. When using AEDs, one electrode pad is placed beside the upper
right sternal border, just below the clavicle, and the other pad is placed just
lateral to the left nipple, with the top of the pad a few inches below the
axilla.
A decrease in time delay between the
last com-pression and the delivery of a shock (the preshock pause) has received
special emphasis in the new guidelines. Stacking shocks increases the time to
next compression, and it has been noted that the first shock is usually
associated with a 90% efficacy. Thus, stacked shocks have been replaced by a recommen-dation
for a single shock, followed by immediate resumption of chest compressions.
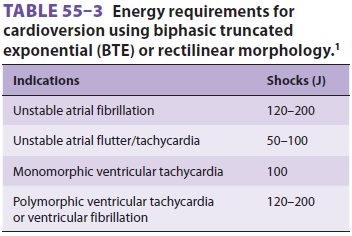
For cardioversion of atrial f briillation (Table 55–3), 120–200
J can be used initially with escalation if needed. For atrial f lutter or
paroxys-mal supraventricular tachycardia (PSVT), an initial energy level of
50–100 J is often adequate. All mono-phasic shocks should start with 200 J.
Ventricular tachycardia, particularly
mono-morphic ventricular tachycardia, responds well to shocks at initial energy
levels of 100 J. For poly-morphic ventricular tachycardia or for ventricular
fibrillation, initial energy can be set at 120–200 J,
depending upon the type of biphasic waveform being used. Stepwise
increases in energy levels should be used if the first shock fails, although
some AEDs operate with a fixed-energy protocol of 150 J with very high success
in terminating ventricular fibrilla-tion (Table 55–3).
Cardioversion should be synchronized with the QRS complex and is recommended
for hemody-namically stable, wide-complex tachycardia requir-ing cardioversion,
PSVT, atrial fibrillation, and atrial flutter. Polymorphic VT should be treated
as VF with unsynchronized shocks.
Invasive Cardiopulmonary Resuscitation
Thoracotomy and open-chest cardiac massage are not part of routine CPR
because of the high inci-dence of severe complications. Nonetheless, these
invasive techniques can be helpful in specific life-threatening circumstances
that preclude effective closed-chest massage. Possible indications include
cardiac arrest associated with penetrating or blunt chest trauma, penetrating
abdominal trauma, severe chest deformity, pericardial tamponade, or pulmonary
embolism.
Intravenous Access
Some resuscitation drugs are fairly well absorbed
following administration through a TT. Lido-caine, epinephrine, atropine,
naloxone, andvasopressin (but not sodium bicarbonate) can be delivered via a
catheter whose tip extends past the TT. Dosages 2–2½ times higher than
recommended for intravenous use, diluted in 10 mL of normal saline or distilled
water, are recommended for adult patients. Even though establishing reliable
intrave-nous access is a high priority, it should not take pre-cedence over
initial chest compressions, airway management, or defibrillation. A preexisting
inter-nal jugular or subclavian line is ideal for venous access during
resuscitation. If there is no central line access, an attempt should be made to
establish peripheral intravenous access in either the antecubi-tal or the
external jugular vein. Peripheral intrave-nous sites are associated with a
significant delay of 1–2 min between drug administration and delivery to the
heart, as peripheral blood flow is drastically reduced during resuscitation.
Administration of drugs given through a peripheral intravenous line should be
followed by an intravenous flush (eg, a 20-mL fluid bolus in adults) and/or
elevation of the extremity for 10–20 s. Establishing central vein access can
potentially cause interruption of CPR but should be considered if an inadequate
response is seen to peripherally administered drugs.If intravenous cannulation
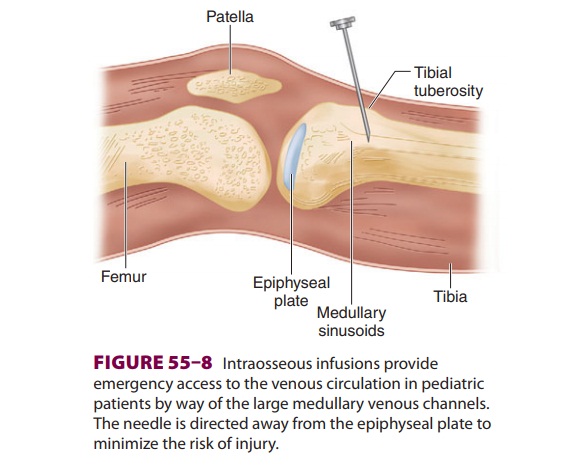
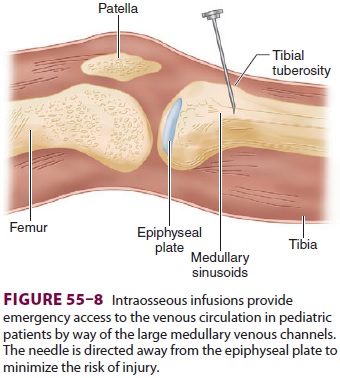
is difficult, an intraosseous infusion can provide emergencyvascular access in
children. The success rate is lower in older children, but even in adults
intraosseous cannulas have been successfully placed in the tibia and in the
distal radius and ulna. A rigid 18-gauge spinal needle with a stylet or a small
bone marrow trephine needle can be inserted into the distal femur or proximal
tibia. If the tibia is chosen, a needle is inserted 2–3 cm below the tibial
tuberosity at a 45° angle away from the epiphyseal plate (Figure
55–8). Once the needle is advanced through the
cortex, it should stand upright without support. Proper place-ment is confirmed
by the ability to aspirate marrow through the needle and a smooth infusion of
fluid.network of venous sinusoids within the medullary cavity of long bones
drains into the systemic cir-culation by way of nutrient or emissary veins.
This route is very effective for administration of drugs, crystalloids,
colloids, and blood and can achieve flow rates exceeding 100 mL/h under
gravity. Much higher flow rates are possible if the fluid is placed under
pressure (eg, 300 mm Hg) with an infu-sion bag. The onset of drug action may be
slightly delayed compared with intravenous or tracheal
administration. The intraosseous route may require a higher dose of some drugs (eg, epinephrine) than recommended for
intravenous administra-tion. The use of intraosseous infusion for induction and
maintenance of general anesthesia, antibiotic therapy, seizure control, and
inotropic support has been described. (Note that most studies have evalu-ated
the placement of intraosseous access in patients with intact hemodynamics or
hypovolemic states, not in cardiac arrest situations.) Because of the risks of
osteomyelitis and compartment syndrome, how-ever, intraosseous infusions should
be replaced by a conventional intravenous route as soon as possible. In addition,
because of the theoretical risk of bone marrow or fat emboli, intraosseous
infusions should be avoided if possible in patients with right-to-left shunts,
pulmonary hypertension, or severe pulmo-nary insufficiency.
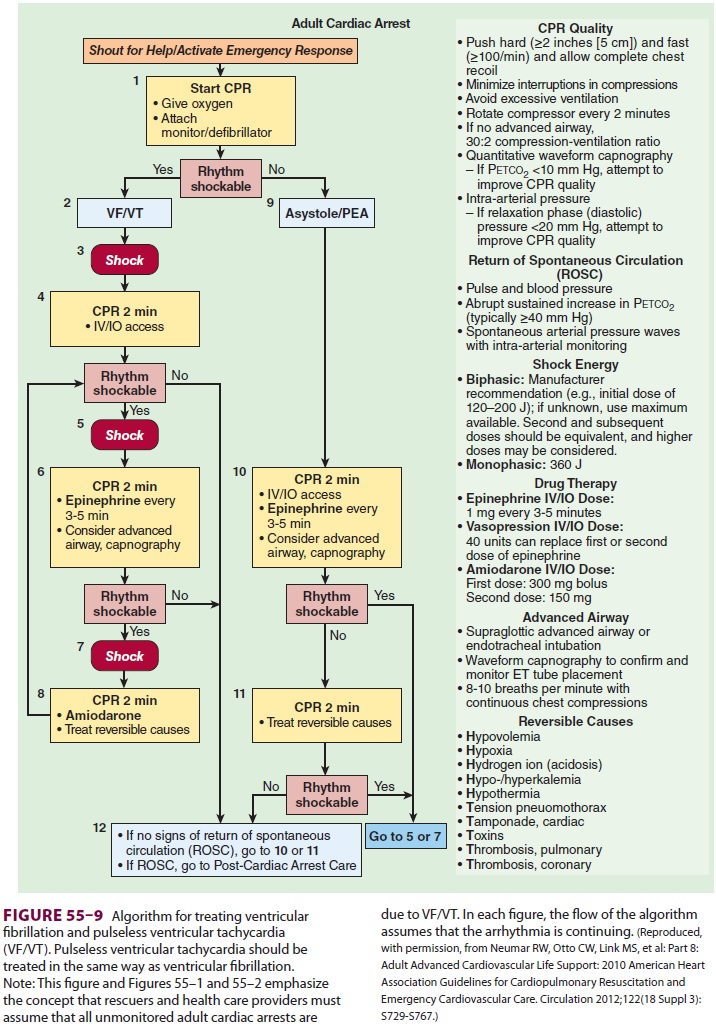
Arrhythmia Recognition
Successful pharmacological and electrical
treatment of cardiac arrest (Figure 55–9)
depends on defini-tive identification of the underlying arrhythmia.
Interpreting rhythm strips in the midst of a resus-citation situation is
complicated by artifacts and variations in monitoring techniques (eg, lead
sys-tems, equipment).
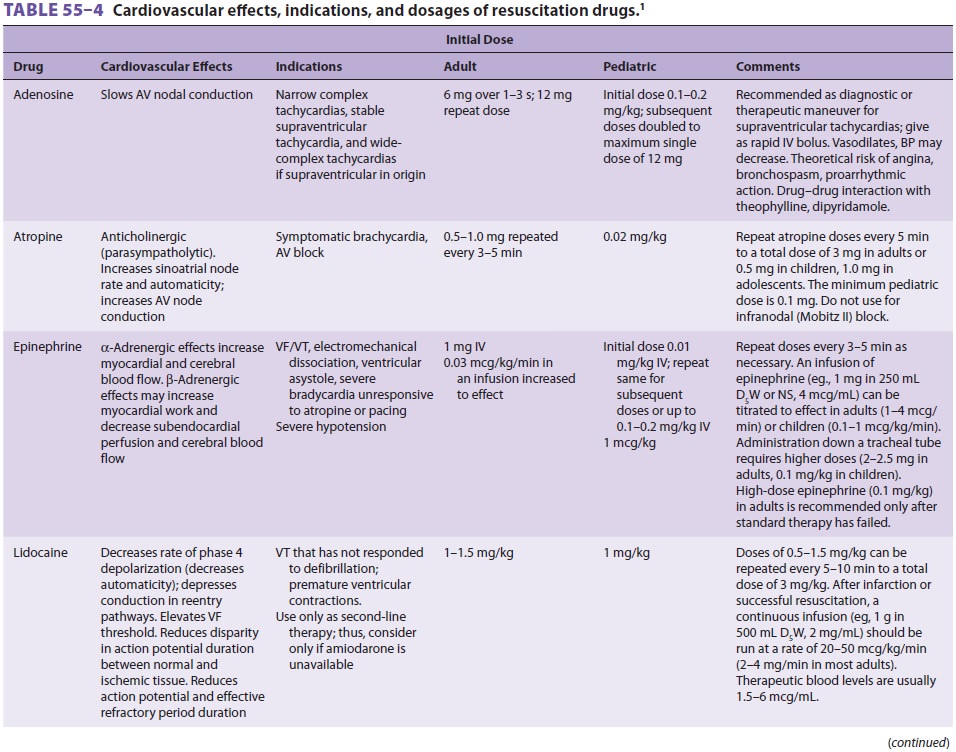
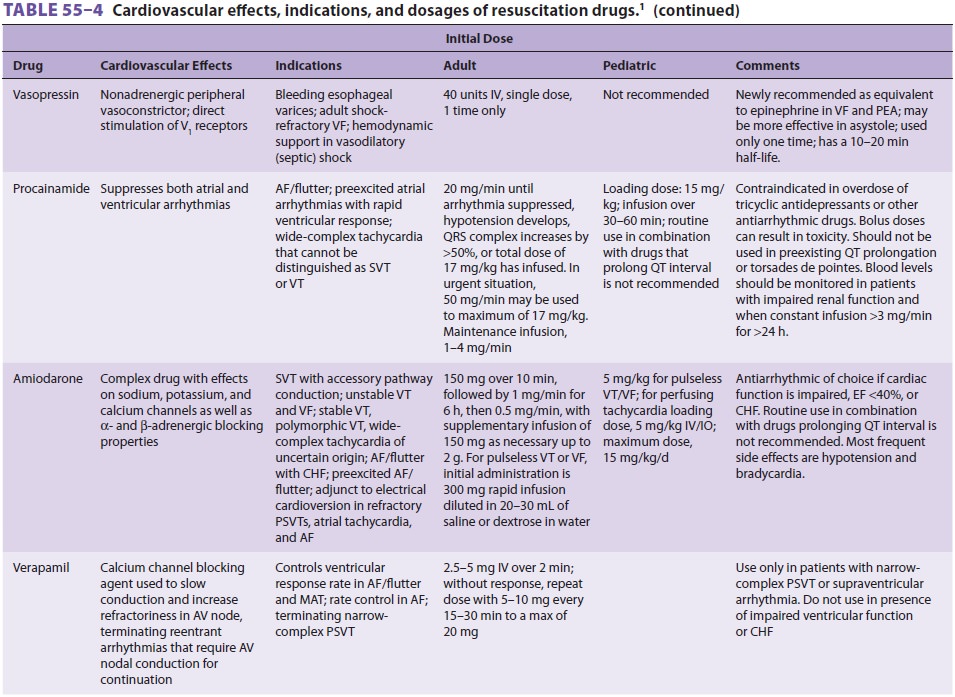
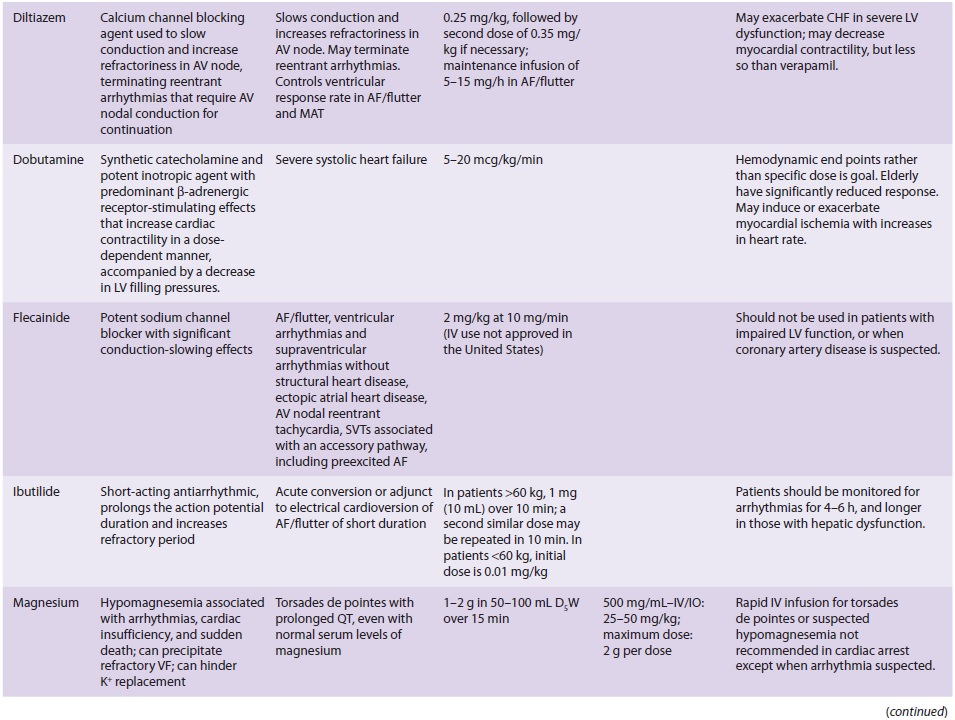
Drug Administration
Many of the drugs administered during CPR have been described elsewhere
in this text. Table 55–4 sum-marizes the cardiovascular
actions, indications, and dosages of drugs commonly used during resuscitation.
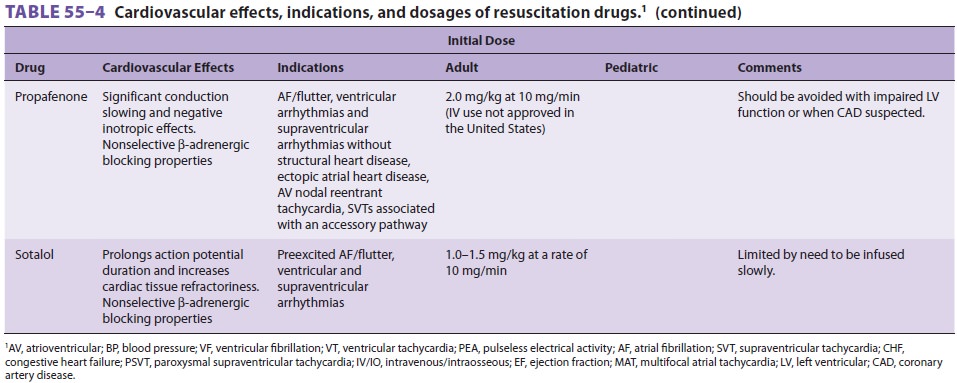
Atropine is not included as a drug for PEA/ asystole in the new CPR-ECC
guidelines; however, its use is retained for symptomatic bradycardia.
Infu-sions of chronotropic drugs (eg, dopamine, epineph-rine, isoproterenol)
can be considered as an alternative to pacing if atropine is ineffective in the
setting of symptomatic bradycardia. Calcium chloride, sodium bicarbonate, and
bretylium are conspicuously absent from this table. Calcium (2–4 mg/kg of the
chloride salt) is helpful in the treatment of documented hypo-calcemia,
hyperkalemia, hypermagnesemia, or a cal-cium channel blocker overdose. When
used, 10% calcium chloride can be given at 2–4 mg/kg every 10 min. Sodium
bicarbonate (0.5–1 mEq/kg) is not recommended in the guidelines and should be
con-sidered only in specific situations such as preexisting metabolic acidosis
or hyperkalemia, or in the treat-ment of tricyclic antidepressant or
barbiturate over-dose. Sodium bicarbonate elevates plasma pH by combining with
hydrogen ions to form carbonic acid, which readily dissociates into carbon
dioxide and water. Because carbon dioxide, but not
bicar-bonate, readily crosses cell membranes and theblood–brain barrier, the
resulting arterial hypercap-nia will cause intracellular tissue acidosis.
Although successful defibrillation is not related to arterial pH, increased intramyocardial
carbon dioxide may reduce the possibility of cardiac resuscitation.
Furthermore, bicarbonate administration can lead to detrimental alterations in
osmolality and the oxygen–hemoglobin dissociation curve. Therefore, effective
alveolar venti-lation and adequate tissue perfusion are the treat-ments of
choice for the respiratory and metabolic acidosis that accompany resuscitation.
Intravenous fluid therapy with either colloid or balanced salt solutions is indicated in patients with intravascular volume depletion (eg, acute blood loss, diabetic ketoacidosis, thermal burns). Dextrose-containing solutions may lead to a hyperosmotic diuresis and may worsen neurological outcome. They should be avoided unless hypoglycemia is suspected.
Likewise, administration of free water (eg, D5W) may
lead to cerebral edema.
Emergency Pacemaker Therapy
Transcutaneous cardiac pacing (TCP) is a
noninva-sive method of rapidly treating arrhythmias caused by conduction
disorders or abnormal impulse. TCP is not routinely recommended in cardiac
arrest. TCP use may be considered to treat asystole, bradycardia caused by
heart block, or tachycardia from a reentrant mechanism. If there is concern
about the use of atro-pine in high-grade block, TCP is always appropriate. If
the patient is unstable with marked bradycardia, TCP should be implemented
immediately while awaiting treatment response to drugs. The pacer unit has
become a built-in feature of some defibrillator models. Disposable pacing
electrodes are usually positioned on the patient in an anterior–posterior
manner. The placement of the negative electrode cor-responds to a V2
electrocardiograph position, whereas the positive electrode is placed on the
left posterior chest beneath the scapula and lateral to the spine. Note that this
positioning does not interfere with pad-dle placement during defibrillation.
Failure to capture may be due to electrode misplacement, poor
elec-trode-to-skin contact, or increased transthoracic impedance (eg,
barrel-shaped chest, pericardial effu-sion). Current output is slowly increased
until the pacing stimuli obtain electrical and mechanical capture. A wide QRS
complex following a pacing spike signals electrical capture, but
mechanical(ventricular) capture must be confirmed by an improving pulse or blood
pressure. Conscious patients may require sedation to tolerate the discom-fort
of skeletal muscle contractions. Transcutaneous pacing can provide effective
temporizing therapy until transvenous pacing or other definitive treatment can
be initiated. TCP has many advantages over transvenous pacing because it can be
used by almost all electrocardiogram providers and can be started quickly and
conveniently at the bedside.
Precordial Thump
The precordial thump is to be considered only in witnessed, monitored
unstable VT when a defibril-lator is not immediately available.
Related Topics