Chapter: Modern Pharmacology with Clinical Applications: Thyroid and Antithyroid Drugs
Biosynthesis, Storage, Secretion, and Metabolism of Thyroid Hormones
BIOSYNTHESIS,
STORAGE, SECRETION, AND METABOLISM OF THYROID HORMONES
Thyroid epithelial cells
synthesize and secrete T4 and T3 and make up the
functional units of thyroid glandular tissue, the thyroid follicles. Thyroid
follicles are hollow vesicles formed by a single layer of epithelial cells that
are filled with colloid. T4,T3,
and iodine are stored in the follicular colloid. T4 and T3
are derived from tyrosyl residues of the protein thyroglobulin (Tg). Thyroid fol-licular cells synthesize and
secrete Tg into the follicular lumen. Thyroid follicular cells also remove
iodide (I ) from the blood and concentrate it within the follicular lumen.
Within the follicles, some of the tyrosyl residues of Tg are iodinated, and a
few specific pairs of iodoty-rosyl residues may be coupled to form T4
and T3. Thus, T4, T3, and iodine (in the form
of iodinated tyrosyl residues) are found within the peptide structure of the Tg
that is stored in the follicular lumen.
The secretion of T4
and T3 requires the uptake of fol-licular contents across the
follicular cell apical mem-brane, the enzymatic release of T4 and T3
from peptide linkage within Tg, and the transport of T4 and T3
across the follicular cell basal membrane to the blood. Several of the steps in
synthesis and secretion of T4 and T3 may be compromised
by iodine deficiency or disease and can be blocked selectively by a variety of
chemicals and drugs.
Requirement for Iodine
A normal rate of thyroid
hormone synthesis depends on an adequate dietary intake of iodine. Iodine is
naturally present in water and soil, although some soils contain very low
amounts. As a result, seafood is a more reliable source of iodine than crop
plants. Approximately 1.6 billion people in more than 100 countries live in
areas where natural sources of dietary iodine intake are mar-ginal or
insufficient. A minimum of 60 g of elemental iodine is required each day for thyroid
hormone syn-thesis, and at least 100 g/day is required to eliminate thyroid
follicular cell hyperplasia and thyroid enlarge-ment (i.e., iodine deficiency
goiter).
Subsequent to the ingestion
of iodine in various forms, I is absorbed by the small intestine and enters the
blood. Two competing pathways are involved in the clearance of I from the
blood: renal filtration into urine and thyroidal uptake.The renal clearance
rate for I (30–50 mL/minute) varies only with the glomerular filtration rate.
However, the thyroidal I clearance rate is autoregulated to main-tain an
absolute thyroidal I uptake rate of approximately 100 g I each day. To
accomplish this, the thyroidal I clearance rate may vary (3 to 100 mL/minute)
depending on the concentration of I in the blood.
Iodide Transport by Follicular Cells and Iodine Trapping Within Follicles
The thyroid follicular cells
transport I across the cell and secrete the precursor protein, Tg, into the
fol-licular lumen. In addition, these cells contain an apical membrane–bound
enzyme, thyroperoxidase (TPO), and the enzymatic machinery to produce hydrogen
per-oxide (H2O2). In the presence of H2O2,
TPO catalyzes the incorporation of I into tyrosyl residues of Tg to form
monoiodotyrosine (MIT) and diiodotyrosine (DIT) and the coupling of these
iodotyrosyl residues to form T4 and T3.
Thyroid follicular cells actively transport iodide into the cell against both a concentration gradient and a neg-ative potential (Fig. 65.1). At the basal (blood side) fol-licular cell membrane, an iodide pump actively transports I- from the extracellular fluid (pertechnetate) into the cytoplasm and concentrates I- within the follicular cell.
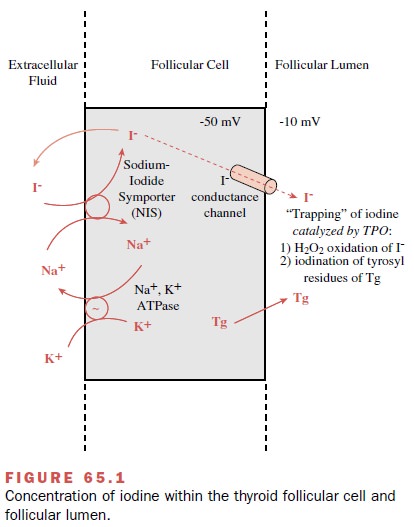
The I- concentration gradient between the thyroid gland
and the blood normally ranges from 25 to 100 and is re-ferred to as the
thyroid–plasma or thyroid–serum ratio. During periods of active stimulation,
the concentration of I- within the follicle may be as high as 250 times that of
the blood. On the luminal side of the apical mem-brane, the I- is rapidly
oxidized in the presence of H2O2 and TPO and incorporated
into the tyrosyl residues in newly formed Tg to form MIT or DIT.
The thyroidal mechanism used
for concentrating I- may also concentrate other monovalent anions, in-cluding
pertechnetate, perchlorate, and thiocyanate, within the follicular lumen.
However, none of these an-ions become incorporated into Tg, although they may
act as a competitive inhibitor of I- transport. The ability of the thyroid
gland to concentrate radioactive pertech-netate makes it a useful agent for
thyroid imaging, since it is concentrated by the thyroid cells without further
metabolism. The perchlorate and thiocyanate discharge tests make use of the
ability of these anions to inhibit I- transport to test for defects in the
incorporation of I- into Tg.
Coupling of Iodotyrosines to Form Iodothyronines
The final step in thyroid
hormone synthesis is the cou-pling of two iodotyrosines
within a single peptide chain of Tg to form the iodothyronine T4 or T3. Both the cou-pling of
two DITs to form T4 and the coupling of a MIT with a DIT to form T3
are catalyzed by the enzyme TPO.
Storage of Thyroid Hormones and Iodine in Colloid
T4, T3,
MIT, and DIT are stored outside the cell in the follicular colloid in peptide
linkage within the Tg mole-cules. In normal humans on an iodine-sufficient
diet, Tg makes up approximately 30% of the mass of the thyroid gland and
represents a 2- to 3-month supply of hormone. The total amount of iodine
contained as T4, T3, MIT, and DIT within Tg varies with
the dietary iodine intake.
Secretion of Thyroid Hormones
The secretion of T4
and T3 is a relatively complex process because T4 and T3
are stored in the peptide structure of Tg within the follicular lumen and
therefore are separated from the pertechnetate and the capillary endothelium by
the thyroid follicular cells.
Endocytosis
The first step in the release
of thyroid hormones from the thyroid gland is through endocytosis of colloid from the follicular lumen into the follicle
cells. This may oc-cur by macropinocytosis or micropinocytosis. Both processes
are stimulated by TSH and result in the up-take of macropinocytotic or micropinocytotic
vesicles that are limited by a single membrane and are filled with colloid
inclusions. These endocytotic vesicles mi-grate from the follicular cell apical
membrane toward the basal membrane. Within a few minutes of their for-mation,
the colloid-containing endocytotic vesicles be-come surrounded by lysosomes
containing glycoside hy-drolases and proteases. The lysosomes eventually fuse
with the endocytotic vesicles to form lysoendosomes. Within the lysoendosomes,
Tg is hydrolyzed to yield peptide fragments, iodoamino acids (MIT and DIT),
iodothyronines (T4 and T3), and other free amino acids.
Once released from Tg, T4 and T3 rapidly diffuse across
the basal plasma membrane into the pertechnetate and eventually into the
circulation. During thyroidal secre-tion, only T4,T3 and
a small amount of I- normally reach the circulation; no Tg, MIT, or DIT
escapes.
The T4 and T3
that are released from the thyroid gland are firmly but reversibly bound to
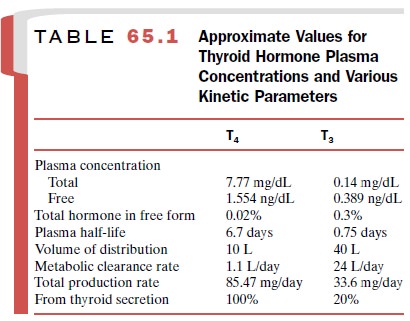
several plasma proteins. More than 99% of the circulating thyroid hor-mone is
protein bound, with only the free hormone available to enter cells (Table
65.1). The amount of T4 or T3 entering the cells and the
ultimate physiological re-sponse are directly related to the plasma
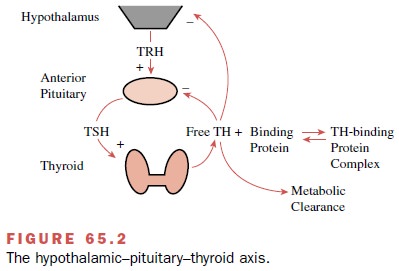
concentrations of free T4 and free T3. It is the
concentrations of free T4 and T3 in the plasma that are
regulated by the HPTA (Fig. 65.2) rather than the total (i.e., free plus
protein-bound) plasma T4 and T3 concentrations.
Thyroxine-binding globulin is the least abundant of the three major transport proteins. Nevertheless, it car-ries about 70% of the circulating T4 and T3 by virtue of its high affinity for the two hormones. Transthyretin, for-merly known as thyroxine-binding prealbumin, binds only about 10 to 15% of the hormones.
Albumin, a pro-tein that has a binding affinity for a multitude of small molecules, has an even lower affinity for T4 and T3 than transthyretin, but the high
plasma albumin concentra-tion results in the binding of about 15 to 20% of the
cir-culating thyroid hormones. Like T4 and T3 bound to
transthyretin, the hormones may dissociate rapidly from albumin to generate
free T4 and free T3. Circulating T4 and T3
are also bound by high-density lipoproteins (HDL). Plasma HDL may carry about
3% of the T4 and 6% of the T3. The physiological
significance of this HDL binding is uncertain, but it may play a role in
targeting thyroid hormone delivery to specific tissues.
The thyroid hormone transport
proteins are not es-sential for hormone action. Rather, they participate in the
maintenance of a steady supply of free hormone to tissues. Because of the
presence of the binding proteins in the plasma, the size of the circulating
thyroid hormone pool is quite large, and both T4 and T3
have very long half-lives in humans (Table 65.1). The total amount of thyroid
hormone bound to plasma proteins is about three times that secreted and
degraded in the course of a single day. Three functions can be postulated for
the thyroid hormone transport proteins: (1) extrathyroidal storage of hormone,
(2) a buffering action, such that effects of acute changes in rates of thyroid
gland secretion or hormone metabolic clearance on plasma concentrations of free
thyroid hormones are minimized, and (3) a hormone-releasing function that allows
the very small free hor-mone pool to be continuously replenished and made
available to cells as intracellular hormone is metabolized. Thus, the large
pools of protein-bound T4 and T3 in the blood act to
stabilize plasma free T4 and free T3 concen-trations and
consequently the intracellular concentra-tions of T4 and T3
and thyroid hormone receptor (TR) occupancy.
Cellular Uptake and Intracellular Binding of T3 to Nuclear Thyroid Hormone Receptors
Free T4 and T3
can enter cells by carrier-mediated facil-itated diffusion or active transport.
After gaining access to the cell interior, T4 may undergo 5’-monodeiodina-tion
to yield T3. The T3 thus mixes with T3
entering the cell from the plasma and binds to nuclear TRs. The specific 5’
-monodeiodinase enzyme and the level of activ-ity vary from tissue to tissue,
as does the contribution of plasma T4 to nuclear TR-bound T3.
Thyroid Hormone Activation and Inactivation by Selenodeiodinases
In humans, the major pathway
in the metabolism of the thyroid hormones consists of the removal of iodine or
deiodination. Three deiodinase isoenzymes, encoded on three distinct genes,
catalyze the reductive deiodination. All three enzymes contain the rare amino
acid seleno-cysteine. The essential trace element selenium therefore plays an
important role in thyroid hormone economy.
The most important pathway
for the metabolism of T4 is monodeiodination. The removal of an
iodide from the outer ring of T4 yields T3. Since the
affinity of nu-clear TRs is much higher for T3 than T4,
outer ring mon-odeiodination of T4 to yield T3 produces a
more active metabolite. Conversely, removal of an iodide from the inner ring of
T4 yields an inactive metabolite, rT3. Both T3
and rT3 may undergo subsequent deiodinations to yield totally deiodinated
thyronine (T0).
Up to 80% of the circulating
T3 originates from deiodination of T4. This is due mainly
to a deiodinase (D1) activity in the liver, where most of the T3
formed is exported into the circulation. Monodeiodination of T4 to
yield T3 is catalyzed by another deiodinase (D2). It appears that D2
catalyzes T3 from T4 for local cellular demands
independent of circulating T3. The third en-zyme involved in the
reductive deiodination of T4, T3, and other
iodothyronines is D3. The sole action of this enzyme is the removal of iodide
from the inner ring of iodothyronines.
The three deiodinases have differing tissue distribu-tions, substrate preferences, and Km values. This arrangement allows for control of thyroid hormone ac-tion at the cellular level. The source and quantity of T3 bound to nuclear TRs may vary among tissues depend-ing on the distributions and relative activities of D1, D2, and D3.
Related Topics