Chapter: Basic & Clinical Pharmacology : Cholinoceptor Blocking Drugs
Basic Pharmacology of the Muscarinic Receptor Blocking Drugs
BASIC
PHARMACOLOGY OF THE MUSCARINIC RECEPTOR BLOCKING DRUGS
Muscarinic
antagonists are sometimes called parasympatholytic because they block the
effects of parasympathetic autonomic dis-charge. However, they do not “lyse”
parasympathetic nerves, and they have some effects that are not predictable
from block of the parasympathetic nervous system. For these reasons, the term
“anti-muscarinic” is preferable.
Naturally
occurring compounds with antimuscarinic effects have been known and used for
millennia as medicines, poisons, and cosmetics. Atropine is the prototype of these drugs. Many similar plant
alkaloids are known, and hundreds of synthetic anti-muscarinic compounds have
been prepared.
Chemistry & Pharmacokinetics
A. Source and Chemistry
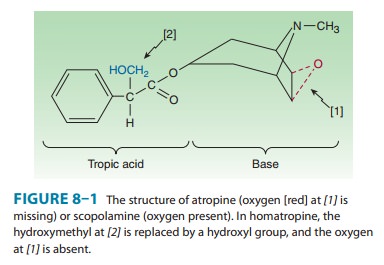
Atropine
and its naturally occurring congeners are tertiary amine alkaloid esters of
tropic acid (Figure 8–1). Atropine (hyoscyamine) is found in the plant Atropa belladonna, or deadly nightshade,
and in Datura stramonium, also known
as jimson-weed (Jamestown weed), sacred Datura, or thorn apple. Scopolamine (hyoscine) occurs in Hyoscyamus niger, or henbane, as the l(−) stereoisomer. Naturally occurring
atropine is l(−)-hyoscyamine, but the
com-pound readily racemizes, so the commercial material is racemic d,l-hyoscyamine. Thel(−)
isomers of both alkaloids are at least100 times more potent than the d(+) isomers.
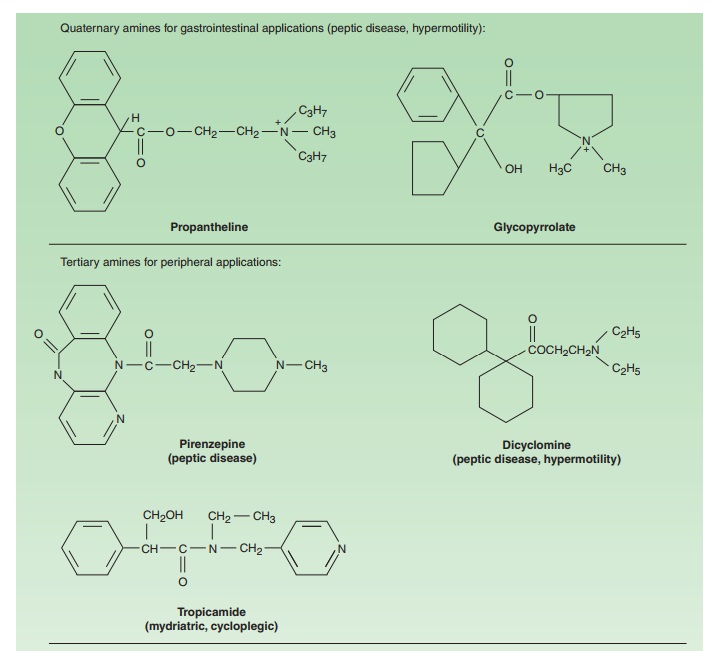
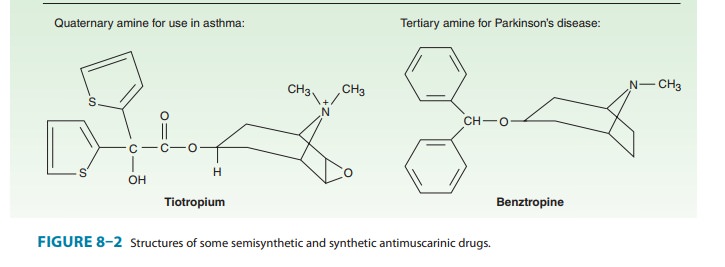
A
variety of semisynthetic and fully synthetic molecules have anti-muscarinic
effects. The tertiary members of these classes (Figure 8–2) are often used for
their effects on the eye or the CNS. Many anti-histaminic , antipsychotic , and
antidepressant drugs have similar
structures and, predictably, significant antimuscarinic effects.
Quaternary
amine antimuscarinic agents (Figure 8–2) have been developed to produce more
peripheral effects and reduced CNS effects.
B. Absorption
Natural
alkaloids and most tertiary antimuscarinic drugs are well absorbed from the gut
and conjunctival membranes. When applied in a suitable vehicle, some (eg,
scopolamine) are even absorbed across the skin (transdermal route). In
contrast, only 10–30% of a dose of a quaternary antimuscarinic drug is absorbed
after oral administration, reflecting the decreased lipid solubility of the
charged molecule.
C. Distribution
Atropine
and the other tertiary agents are widely distributed in the body. Significant
levels are achieved in the CNS within 30 min-utes to 1 hour, and this can limit
the dose tolerated when the drug is taken for its peripheral effects. Scopolamine
is rapidly and fully distributed into the CNS where it has greater effects than
most other antimuscarinic drugs. In contrast, the quaternary derivatives are
poorly taken up by the brain and therefore are relatively free—at low doses—of
CNS effects.
D. Metabolism and Excretion
After
administration, the elimination of atropine from the blood occurs in two
phases: the t½ of the rapid
phase is 2 hours and that of the slow phase is approximately 13 hours. About
50% of the dose is excreted unchanged in the urine. Most of the rest appears in
the urine as hydrolysis and conjugation products. The drug’s effect on
parasympathetic function declines rapidly in all organs except the eye. Effects
on the iris and ciliary muscle persist for ≥ 72 hours.
Pharmacodynamics
A. Mechanism of Action
Atropine
causes reversible (surmountable) blockade
of cholinomimetic actions at muscarinic receptors; that is, block-ade by
a small dose of atropine can be overcome by a larger con-centration of
acetylcholine or equivalent muscarinic agonist. Mutation experiments suggest
that aspartate in the third trans-membrane segment of the heptahelical receptor
forms an ionic bond with the nitrogen atom of acetylcholine; this amino acid is
also required for binding of antimuscarinic drugs. When atropine binds to the
muscarinic receptor, it prevents actions such as the release of inositol
trisphosphate (IP3) and the inhibition of adeny-lyl cyclase that are
caused by muscarinic agonists . Muscarinic antagonists were traditionally
viewed as neutral com-pounds that occupied the receptor and prevented agonist
binding. Recent evidence indicates that muscarinic receptors are
constitu-tively active, and most drugs that block the actions of acetylcho-line
are inverse agonists that shift the
equilibrium to the inactive state of the receptor. Muscarinic blocking drugs
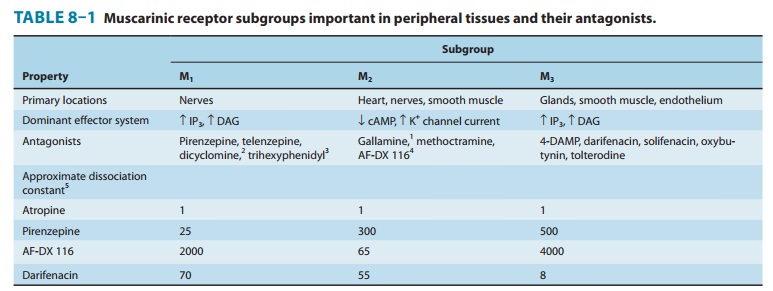
that are inverse agonists include atropine, pirenzepine, trihexy-phenidyl,
AF-DX 116, 4-DAMP, ipratropium, glycopyrrolate, and a methyl derivative of
scopolamine (Table 8–1).
The
effectiveness of antimuscarinic drugs varies with the tissue and with the
source of agonist. Tissues most sensitive to atropine are the salivary,
bronchial, and sweat glands. Secretion of acid by the gastric parietal cells is
the least sensitive. In most tissues, antimusca-rinic agents block exogenously
administered cholinoceptor agonists more effectively than endogenously released
acetylcholine.
Atropine
is highly selective for muscarinic receptors. Its potency at nicotinic
receptors is much lower, and actions at non-muscarinic receptors are generally
undetectable clinically.
Atropine
does not distinguish among the M1, M2, and M3
subgroups of muscarinic receptors. In contrast, other antimuscar-inic drugs are
moderately selective for one or another of these subgroups (Table 8–1). Most
synthetic antimuscarinic drugs are considerably less selective than atropine in
interactions with non-muscarinic receptors. For example, some quaternary amine
anti-muscarinic agents have significant ganglion-blocking actions, and others
are potent histamine receptor blockers. The antimuscarinic effects of other
agents, eg, antipsychotic and antidepressant drugs, have been mentioned. Their
relative selectivity for muscarinic receptor subtypes has not been defined.
B. Organ System Effects
1. Central nervous system—In the doses usually used, atropinehas minimal stimulant effects on the CNS, especially the parasympa-thetic medullary centers, and a slower, longer-lasting sedative effect on the brain. Scopolamine has more marked central effects, producing drowsiness when given in recommended dosages and amnesia in sensi-tive individuals. In toxic doses, scopolamine, and to a lesser degree atropine, can cause excitement, agitation, hallucinations, and coma.
The
tremor of Parkinson’s disease is reduced by centrally acting antimuscarinic
drugs, and atropine—in the form of belladonna extract—was one of the first
drugs used in the therapy of this dis-ease. As discussed, parkinsonian tremor
and rigidity seem to result from a relative
excess of cholinergic activity because of a deficiency of dopaminergic activity
in the basal ganglia-stria-tum system. The combination of an antimuscarinic
agent with a dopamine precursor drug (levodopa) can sometimes provide more
effective therapy than either drug alone.
Vestibular
disturbances, especially motion sickness, appear to involve muscarinic
cholinergic transmission. Scopolamine is often effective in preventing or reversing
these disturbances.
2. Eye—The pupillary
constrictor muscle (see Figure 6–9) dependson muscarinic cholinoceptor
activation. This activation is blocked by topical atropine and other tertiary
antimuscarinic drugs and results in unopposed sympathetic dilator activity and mydriasis (Figure 8–3). Dilated pupils
were considered cosmetically desirable during the Renaissance and account for
the name belladonna (Italian, “beauti-ful lady”) applied to the plant and its
active extract because of the use of the extract as eye drops during that time.
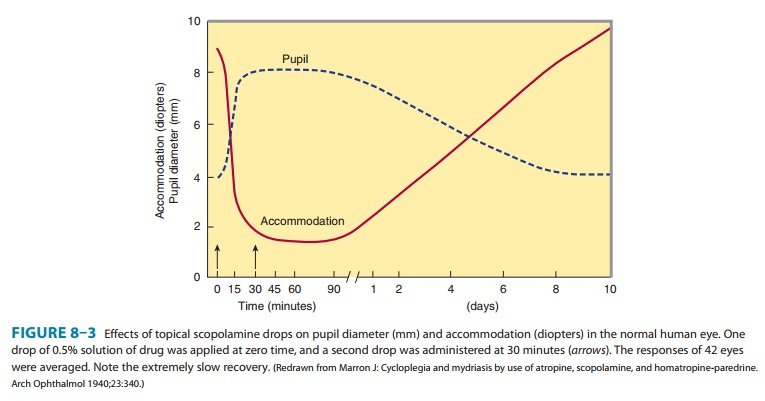
The
second important ocular effect of antimuscarinic drugs is to weaken contraction
of the ciliary muscle, or cycloplegia.
Cycloplegia results in loss of the ability to accommodate; the fully
atropinized eye cannot focus for near vision (Figure 8–3).
Both
mydriasis and cycloplegia are useful in ophthalmology. They are also
potentially hazardous, since acute glaucoma may be induced in patients with a
narrow anterior chamber angle.
A
third ocular effect of antimuscarinic drugs is to reduce lacri-mal secretion.
Patients occasionally complain of dry or “sandy” eyes when receiving large
doses of antimuscarinic drugs
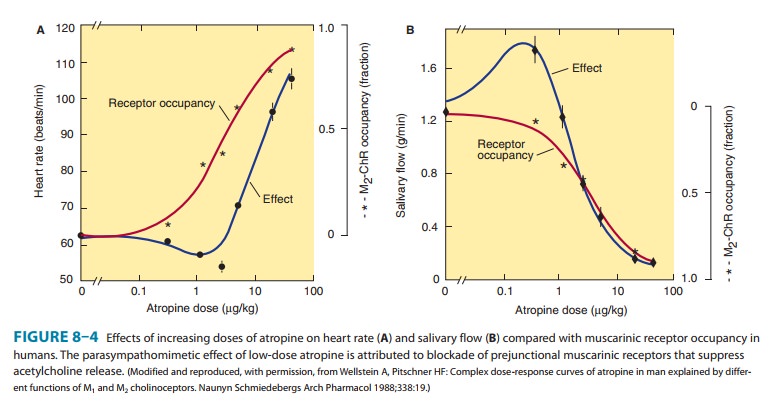
3. Cardiovascular system—The sinoatrial node is
very sensi-tive to muscarinic receptor blockade. Moderate to high therapeu-tic
doses of atropine cause tachycardia in the innervated and spontaneously beating
heart by blockade of vagal slowing. However, lower doses often result in
initial bradycardia before theeffects of peripheral vagal block become manifest
(Figure 8–4). This slowing may be due to block of prejunctional M1
receptors (autoreceptors, see Figure 6–3) on vagal postganglionic fibers that
normally limit acetylcholine release in the sinus node and other tissues. The
same mechanisms operate in the atrioventricular node; in the presence of high
vagal tone, atropine can significantly reduce the PR interval of the
electrocardiogram by blocking mus-carinic receptors in the atrioventricular
node. Muscarinic effects on atrial muscle are similarly blocked, but these
effects are of no clinical significance except in atrial flutter and
fibrillation. The ventricles are less affected by antimuscarinic drugs at
therapeutic levels because of a lesser degree of vagal control. In toxic
concen-trations, the drugs can cause intraventricular conduction block that has
been attributed to a local anesthetic action.
Most
blood vessels, except those in thoracic and abdominal viscera, receive no
direct innervation from the parasympathetic system. However, parasympathetic
nerve stimulation dilates coro-nary arteries, and sympathetic cholinergic
nerves cause vasodilation in the skeletal muscle vascular bed . Atropine can
block this vasodilation. Furthermore, almost all vessels contain endothelial
muscarinic receptors that mediate vasodilation . These receptors are readily
blocked by antimuscarinic
drugs.
At toxic doses, and in some individuals at normal doses, antimuscarinic agents
cause cutaneous vasodilation, especially in the upper portion of the body. The
mechanism is unknown.
The
net cardiovascular effects of atropine in patients with nor-mal hemodynamics
are not dramatic: tachycardia may occur, but there is little effect on blood
pressure. However, the cardiovascular effects of administered direct-acting
muscarinic agonists are easily prevented.
4. Respiratory system—Both smooth muscle and secretoryglands of the airway receive vagal innervation and contain muscarinic receptors. Even in normal individuals, administration of atropine can cause some bronchodilation and reduce secretion. The effect ismore significant in patients with airway disease, although the anti-muscarinic drugs are not as useful as the β-adrenoceptor stimulants in the treatment of asthma . The effectiveness of nonselective antimuscarinic drugs in treating chronic obstructive pulmonary disease (COPD) is limited because block of autoin-hibitory M2 receptors on postganglionic parasympathetic nerves can oppose the bronchodilation caused by block of M3 receptors on airway smooth muscle. Nevertheless, antimuscarinic agents are valuable in some patients with asthma or COPD.
Antimuscarinic
drugs are frequently used before the adminis-tration of inhalant anesthetics to
reduce the accumulation of secretions in the trachea and the possibility of
laryngospasm.
5. Gastrointestinal tract—Blockade of muscarinic
receptorshas dramatic effects on motility and some of the secretory func-tions
of the gut. However, even complete muscarinic block cannot totally abolish
activity in this organ system, since local hormones and noncholinergic neurons
in the enteric nervous system also
modulate gastrointestinal function. As in other tissues, exogenously administered
muscarinic stimulants are more effectively blocked than are the effects of
parasympathetic (vagal) nerve activity. The removal of autoinhibition, a
negative feedback mechanism by which neural acetylcholine suppresses its own
release, might explain the lower efficacy of antimuscarinic drugs against the
effects of endogenous acetylcholine.
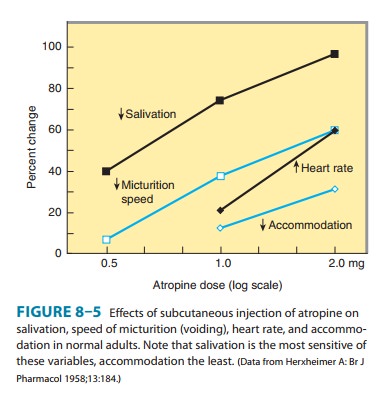
Antimuscarinic
drugs have marked effects on salivary secretion; dry mouth occurs frequently in
patients taking antimuscarinic drugs for Parkinson’s disease or urinary
conditions (Figure 8–5). Gastric secretion is blocked less effectively: the
volume and amount of acid, pepsin, and mucin are all reduced, but large doses
of atropine may be required. Basal secretion is blocked more effectively than
that stimulated by food, nicotine, or alcohol. Pirenzepine and a more potent
analog, telenzepine, reduce gastric acid secretion with fewer adverse effects
than atropine and other less selective agents. This was thought to result from
a selective blockade of excitatory M1 muscar-inic receptors on vagal
ganglion cells innervating the stomach, as suggested by their high ratio of M1
to M3 affinity (Table 8–1). However, carbachol was found to
stimulate gastric acid secretion in animals with M1 receptors
knocked out; M3 receptors were impli-cated and pirenzepine opposed
this effect of carbachol, an indication that pirenzepine is selective but not
specific for M1 receptors. The mechanism of vagal regulation of
gastric acid secretion likely involves multiple muscarinic receptor-dependent
pathways. Pirenzepine and telenzepine are investigational in the USA.
Pancreatic and intestinal secretion are little affected by atropine; these
processes are primarily under hormonal rather than vagal control.
Gastrointestinal
smooth muscle motility is affected from the stomach to the colon. In general,
the walls of the viscera are relaxed, and both tone and propulsive movements
are diminished. Therefore, gastric emptying time is prolonged, and intestinal
tran-sit time is lengthened. Diarrhea due to overdosage with
parasym-pathomimetic agents is readily stopped, and even diarrhea caused by
nonautonomic agents can usually be temporarily controlled. However, intestinal
“paralysis” induced by antimuscarinic drugs is temporary; local mechanisms
within the enteric nervous system usually reestablish at least some peristalsis
after 1–3 days of anti-muscarinic drug therapy.
6. Genitourinary tract—The antimuscarinic
action of atropineand its analogs relaxes smooth muscle of the ureters and
bladder wall and slows voiding (Figure 8–5). This action is useful in the
treatment of spasm induced by mild inflammation, surgery, and certain
neurologic conditions, but it can precipitate urinary reten-tion in men who
have prostatic hyperplasia (see following section, Clinical Pharmacology of the
Muscarinic Receptor-Blocking Drugs). The antimuscarinic drugs have no
significant effect on the uterus.
7. Sweat glands—Atropine suppresses
thermoregulatory sweat-ing. Sympathetic cholinergic fibers innervate eccrine
sweat glands, and their muscarinic receptors are readily accessible to
antimusca-rinic drugs. In adults, body temperature is elevated by this effect
only if large doses are administered, but in infants and children even ordinary
doses may cause “atropine fever.”
Related Topics