Chapter: Essential Anesthesia From Science to Practice : Applied physiology and pharmacology : Anesthesia and other systems
Anesthesia and The brain
Anesthesia and other systems
If you
are reading this, you are not a neurologist, gastroenterologist, hepatologist,
nephrologist, or hematologist. Yet, anesthesiologists need to worry about some
features and functions of the stomach, liver, kidneys, blood, and particularly
the brain. Here is a short perspective on the why and how.
The brain
General
anesthesia is, ultimately, about putting the central nervous system (CNS) to
sleep. We choose this or that agent in an effort to optimize the patient’s
intra-operative course, but in reality the nuances of the different agents make
little difference a few days after minor surgery in a healthy patient. However,
in the patient with intracranial pathology, a thorough understanding of
neurophysiol-ogy and the implications of anesthesia take center stage. Because
we do not know which patients have undiagnosed cerebral aneurysms or tumors, we
like to apply our understanding to all patients.
The
brain is an amazing organ. Despite weighing only about 1.3 kg, just 2% of total
body weight, it receives 15% of the cardiac output and consumes 20% of the
oxygen used by the body and watches over all of the body! Formulating some
mental models of this metabolic workhorse will help to explain its dynamic
workings. Conveniently, the spinal cord behaves physiologically similar to the
brain.
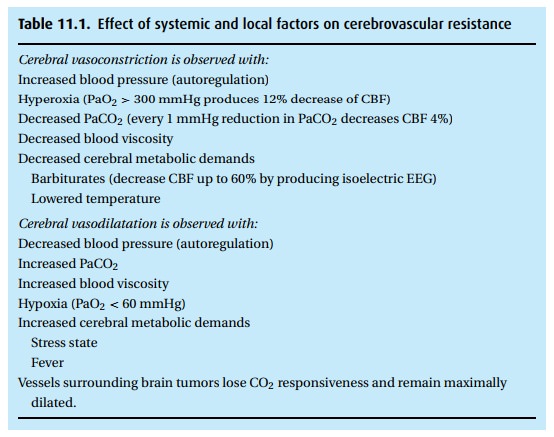
Compared to other organs, cerebral hemodynamics have both similarities and unique features. Numerous factors affect the cerebral vascular system (Table 11.1). The brain autoregulates cerebral blood flow (CBF) to maintain it stable at cere-bral perfusion pressures (CPP) between 65 mmHg and 150 mmHg. But similar to virtually all other organs, it also couples flow to metabolism to assure active areas of the brain receive enough oxygen and glucose to sustain their activities. The cerebral vascular network, curiously, has few alpha-1 receptors. This makes phenylepherine a preferred choice for correcting hypotension without constric-tion cerebral vessels. Opposite to the pulmonary artery’s response, brain vascular responsiveness to hypercarbia causes vasodilatation while hypocarbia produces vasoconstriction and, in extreme cases, can produce cerebral ischemia.
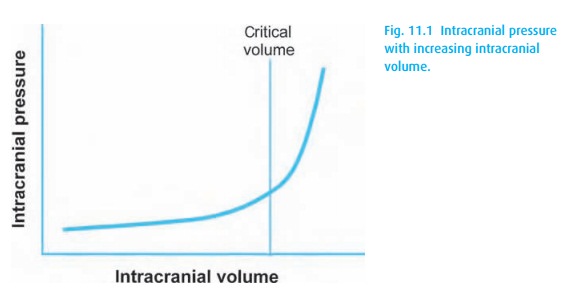
The
skull rigidly constrains the volume of the intracranial space and its three
constituents: brain tissue (1100 g or mL), blood (75 mL), and cerebrospinal
fluid (150 mL). The falx cerebri divides the brain into a left and right
hemisphere, while the tentorium cerebelli separates the cerebellum from the
rest. If any of the brain components increases in volume, either the others
must shrink by a similar amount, or the intracranial pressure (ICP) increases
(Fig. 11.1). This increased pressure may
manifest as papilledema on fundoscopic examination, and as nar-rowed ventricles
or midline shift on an imaging study. Clinical signs include nau-sea, vomiting,
ataxia, altered mental status or the seldom seen Cushing’s triad1 of bradycardia, hypertension and
bradypnea.
Intracranial hypertension poses a significant threat. As the intracranial pressure (ICP) increases beyond a critical point, blood flow to the brain decreases. However, the brain has no stored oxygen. It withstands limited ischemic exposures only by increasing its blood flow or increasing its oxygen extraction from hemoglobin. The brain is a metabolic engine that uses only glucose (or ketones) and oxygen for energy. Sixty percent of the energy used by the brain is spent on perform-ing electrophysiologic functions and 40% on preserving cellular integrity. Thus, defending cerebral perfusion and oxygen delivery are intrinsic to the management of all intracranial masses and elevated ICP. As with all organs, perfusion depends on the pressure difference across the organ:
CPP = MAP − CVP or ICP
where
CPP is cerebral perfusion pressure; MAP, mean arterial pressure; CVP, central
venous pressure; ICP, intracranial pressure (normal mean <15 mmHg). Thus CPP depends on both arterial
blood pressure, and the higher of CVP or ICP.
When
intracranial hypertension continues to rise, the increasing pressure on the
brain must eventually “pop off” into another area. This spontaneous
decom-pression is termed “herniation” and can occur via transtentorial, uncal,
subfal-cine, across the foramen magnum (tonsillar) or out of the skull, when a
fracture offers an opening. Tonsillar herniation pushes the brainstem through
the fora-men magnum, a life-threatening emergency. Herniation is a critical
event, not simply because of the implications of local ischemia – from which a
recovery may be possible – but also because with herniation, sheer forces
produce irreparable mechanical disruption.
With
general anesthesia, we aim to produce a sleeping, well perfused and oxy-genated
brain. Unfortunately, we possess little information about what is actually
happening in the brain and are left with doing our best by using our
under-standing of how the seat of the soul works. For example, we know that the
EEG begins to demonstrate an ischemic pattern when the CBF decreases below
about 20 mL/100 g brain/min, a reduction of over 50% from its normal 50 mL/100
g brain/min perfusion. Hence, hypotension must be treated even in the absence
of cardiac ischemia.
In the
presence of intracranial pathology, we intentionally address each of the
intracerebral volumes to optimize the intra-operative course. We lower the
blood volume by placing the patient in a slightly head-up position to
facilitate venous drainage. Barbiturates given for induction cause an
isoelectric EEG (always, but
We
usually avoid ketamine and halothane because they increase CBF and
dra-matically increase ICP. We may induce mild hyperventilation to produce
arterial vasoconstriction. Under specific circumstances, we might have to
remove CSF peri-operatively via a ventriculostomy or spinal drain. In the
presence of edema or a large mass, we might use steroids and diuretics to
reduce the interstitial vol-ume and, through oxygen free radical scavenging,
protect the brain from ischemic insult. Should the ICP be high, we must defend
CPP, for example by increasing the mean arterial pressure with phenylepherine.
An
aneurysm or arteriovenous malformation challenges us to maintain sta-ble
pressures across the vascular wall by balancing the ICP against the MAP. We
might lower temperature when we anticipate regional ischemic events as can
Otherwise, we work to keep patients warm. The potent inhalational anesthet-ics
all uncouple metabolism-flow autoregulation, causing a decreased metabolic rate
but increasing the CBF. Hence, we use the halogenated vapors in modest
concentrations during intracranial surgery.
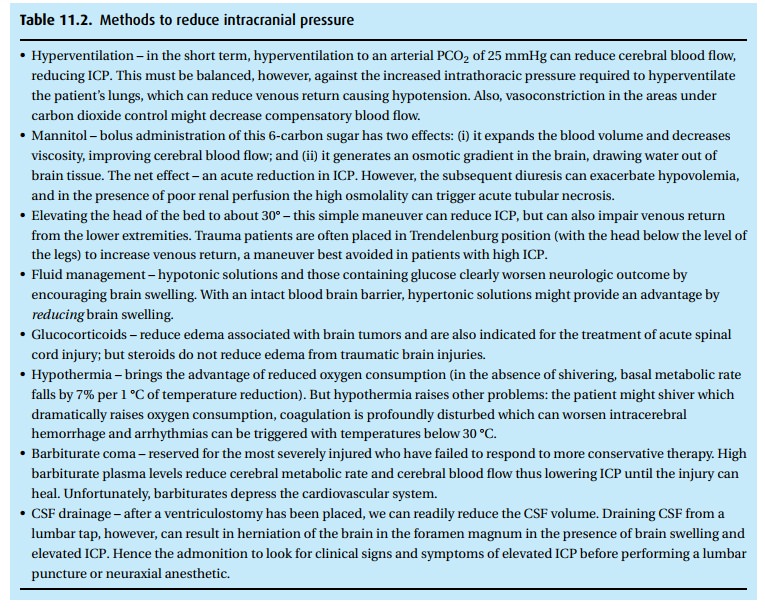
Consider
how one might approach a trauma patient with both arterial hypoten-sion and
increased ICP from a subdural hematoma (SDH). We will work feverishly to
increase his MAP but must also strive to reduce ICP (Table 11.2). We treat low blood pressure in a trauma
patient with the infusion of fluids and, as mentioned above, intravenous
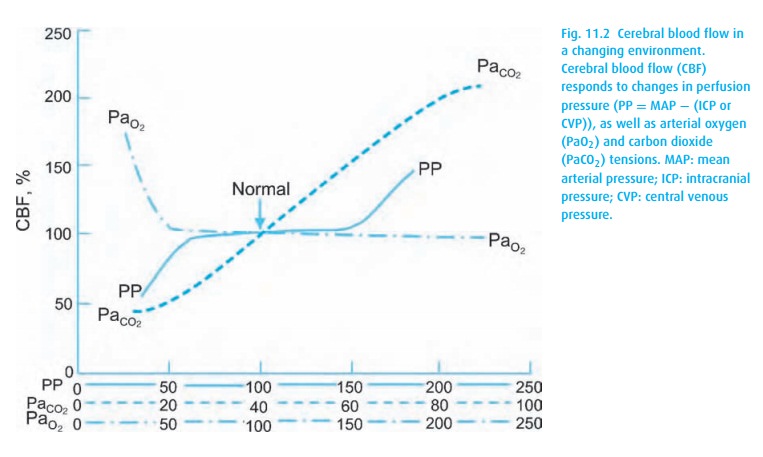
phenylepherine. In addition to CPP, arterial oxygen and car-bon dioxide
tensions affect cerebral blood flow and therefore ICP (Fig. 11.2). We might acutely manipulate PaCO2
in an effort to reduce ICP in the short term; how-ever, aggressive
hyperventilation to decrease ICP can worsen outcome, probably because it can
decrease CBF.
Until
the last decade of the twentieth century, the brain remained an organ that
could not be easily monitored. We had to be guided by changes in heart rate,
blood pressure, urine output, and the patient’s motor response. Today, we
monitor raw and processed EEG, e.g., BIS® monitoring, Aspect Medical, to aid us
in titrating our drugs, avoiding and treating cerebral ischemia and reducing
intra-operative awareness.
Related Topics