Chapter: Plant Biochemistry: Secondary metabolites fulfill specific ecological functions in plants
Alkaloids comprise a variety of heterocyclic secondary metabolites
Alkaloids comprise a variety of heterocyclic secondary metabolites
Alkaloids belong to a group of secondary metabolites that are synthesized from amino acids and contain one or several N atoms as constituents of heterocycles. Many of these alkaloids act as defense compounds against ani-mals and microorganisms. Since alkaloids usually are bases, they are stored in the protonated form, mostly in the vacuole which is acidic. Since ancient times humans have used alkaloids in the form of plant extracts as poisons, stimulants, and narcotics, and, last but not least, as medicine. In 1806 the pharmacy assistant Friedrich Wilhelm Sertürner isolated morphine from poppy seeds. Another 146 years had to pass before the structure of mor-phine was finally resolved in 1952. More than 10,000 alkaloids of very dif-ferent structures are now known. Their biosynthesis pathways are very diverse, to a large extent still not known, and will not be discussed here.
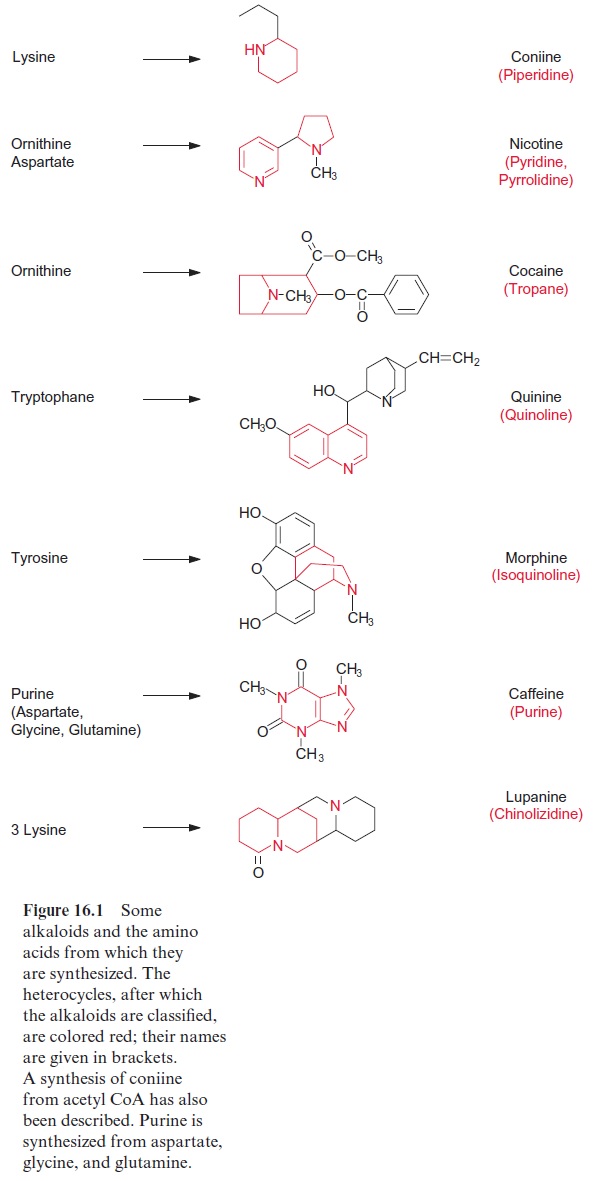
Figure 16.1 shows a small selection of important alkaloids. Alkaloids are classified according to their heterocycles. Coniine, a piperidine alkaloid, is a very potent poison in hemlock. Socrates died when he was forced to drink this poison. Nicotine, which also is very toxic, contains a pyridine and a pyrrolidine ring. It is synthesized in the roots of tobacco plants and is car-ried along with the xylem sap into the stems and leaves. Nicotine sulfate, a by-product of the tobacco industry, is used as a very potent insecticide (e.g., for fumigating greenhouses). There is no insect known to be resistant to nicotine. Genetically modified tobacco plants where the nicotine content was decreased by 96% were shown to be highly infested by the caterpil-lar Maduca sexta. Cocaine, the well-known narcotic, contains tropane as a heterocycle, in which the N atom is a constituent of two rings. A further well-known tropane alkaloid is atropine (formula not shown), a poison accu-mulated in deadly nightshade (Atropa belladonna). In low doses, it dilates the pupils of the eye and is therefore used in medicine for eye examination. Cleopatra allegedly used extracts containing atropine to dilate her pupils to appear more attractive. Quinine, a quinoline alkaloid from the bark of Chinchuna officinalis growing in South America, was known by the Spanish conquerors to be an antimalarial drug. The isoquinoline alkaloid morphine is an important pain killer and is also a precursor for the synthesis of heroin. Caffeine, the stimulant of coffee, has purine as the heterocycle. Chinolizidin alkaloids, such as lupinin and lupanin, which primarily accumulate in varie-ties of lupines, are synthesized from three lysine molecules. Due to the toxic-ity of these compounds sheep frequently die in the autumn from eating too much lupine seed. Pyrrolizidin alkaloids, such assenecionin (formula not shown) are synthesized by plants to combat herbivores. These compounds, however, are not harmful to certain specifically adapted herbivores, which accumulate them and thus render themselves poisonous towards predators, parasitoids and pathogens.
In order to search for new medicines, large numbers of plants are being analyzed for their secondary metabolite contents. One result is the alka-loid taxol, isolated from the yew tree Taxus brevifolia, now used for can-cer treatment. Derivatives of the alkaloid camptothezine from the Chinese “happy tree” Camptotheca acuminata are also being clinically tested as can-cer therapeutics. The search for new medicines against malaria and viral infections continues. Since large quantities of pharmacologically interesting compounds often cannot be gained from plant material, attempts are being made with the aid of genetic engineering either to increase production in the corresponding plants or to transfer the plant genes into microorganisms in order to use the latter for production.
Related Topics