Chapter: Medical Physiology: Fetal and Neonatal Physiology
Adjustments of the Infant to Extrauterine Life
Adjustments of the Infant to Extrauterine Life
Onset of Breathing
The most obvious effect of birth on the baby is loss of the placental connection with the mother and, there-fore, loss of this means of metabolic support. One of the most important immediate adjustments required of the infant is to begin breathing.
Cause of Breathing at Birth. After normal delivery from amother who has not been depressed by anesthetics, the child ordinarily begins to breathe within seconds and has a normal respiratory rhythm within less than 1 minute after birth. The promptness with which the fetus begins to breathe indicates that breathing is initiated by sudden exposure to the exterior world, probably result-ing from (1) a slightly asphyxiated state incident to the birth process, but also from (2) sensory impulses that originate in the suddenly cooled skin. In an infant who does not breathe immediately, the body becomes progressively more hypoxic and hypercapnic, which provides additional stimulus to the respiratory center and usually causes breathing within an additional minute after birth.
Delayed or Abnormal Breathing at Birth—Danger of Hypoxia. Ifthe mother has been depressed by a general anesthetic during delivery, which at least partially anesthetizes the fetus as well, the onset of respiration is likely to be delayed for several minutes, thus demonstrating the importance of using as little anesthesia as feasible. Also, many infants who have had head trauma during deliv-ery or who undergo prolonged delivery are slow to breathe or sometimes do not breathe at all. This can result from two possible effects: First, in a few infants, intracranial hemorrhage or brain contusion causes a concussion syndrome with a greatly depressed respira-tory center. Second, and probably much more impor-tant, prolonged fetal hypoxia during delivery can cause serious depression of the respiratory center.
Hypoxia frequently occurs during delivery because of (1) compression of the umbilical cord; (2) premature separation of the placenta; (3) excessive contraction of the uterus, which can cut off the mother’s blood flow to the placenta; or (4) excessive anesthesia of the mother, which depresses oxygenation even of her blood.
Degree of Hypoxia That an Infant Can Tolerate. In an adult,failure to breathe for only 4 minutes often causes death, but a neonate often survives as long as 10 minutes of failure to breathe after birth. Permanent and very serious brain impairment often ensues if breathing is delayed more than 8 to 10 minutes. Indeed, actual lesions develop mainly in the thalamus, in the inferior colliculi, and in other brain stem areas, thus permanently affecting many of the motor functions of the body.
Expansion of the Lungs at Birth. At birth, the walls of thealveoli are at first collapsed because of the surface tension of the viscid fluid that fills them. More than 25 mm Hg of negative inspiratory pressure in the lungs is usually required to oppose the effects of this surface tension and to open the alveoli for the first time. But once the alveoli do open, further respiration can be effected with relatively weak respiratory movements. Fortunately, the first inspirations of the normal neonate are extremely powerful, usually capable of creating as much as 60 mm Hg negative pressure in the intrapleural space.
Figure 83–3 shows the tremendous negative intrapleural pressures required to open the lungs at the onset of breathing. At the top is shown the pres-sure-volume curve (“compliance” curve) for the first breath after birth. Observe, first, the lower part of the curve beginning at the zero pressure pointand moving to the right. The curve shows that the volume of air in the lungs remains almost exactly zero until the negative pressure has reached -40 centimeters water (-30 mm Hg). Then, as the negative pressure increases to -60 centimeters of water, about 40 milliliters of air enters the lungs. To deflate the lungs, considerable pos-itive pressure, about +40 centimeters of water, is required because of viscous resistance offered by the fluid in the bronchioles.
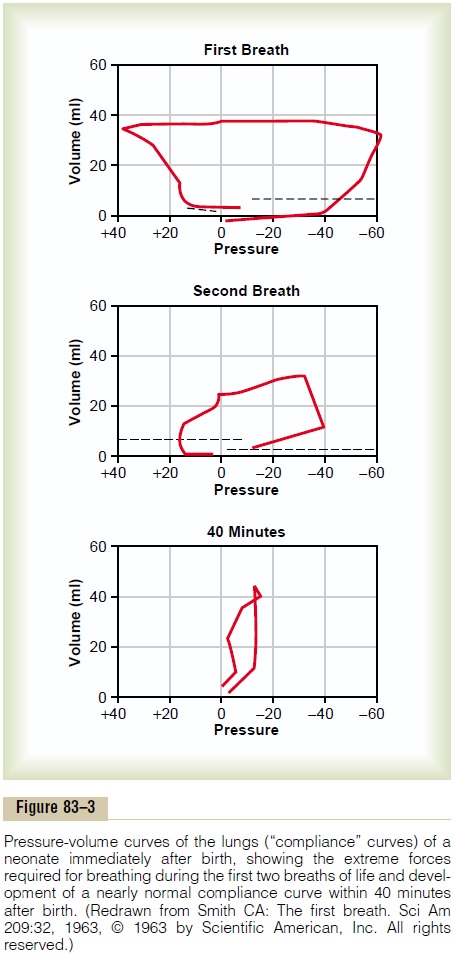
Note that the second breath is much easier, with far less negative and positive pressures required. Breathing does not become completely normal until about 40 minutes after birth, as shown by the third compliance curve, the shape of which compares favorably with that for the normal adult.
Respiratory Distress Syndrome Caused When Surfactant Secretion Is Deficient. A small number of infants, especially pre-mature infants and infants born of diabetic mothers, develop severe respiratory distress in the early hours to the first several days after birth, and some die within the next day or so. The alveoli of these infants at death contain large quantities of proteinaceous fluid, almost as if pure plasma had leaked out of the capillaries into the alveoli. The fluid also contains desquamated alveo-lar epithelial cells. This condition is called hyaline mem-brane disease because microscopic slides of the lungshow the material filling the alveoli to look like a hyaline membrane.
One of the most characteristic findings in respiratory distress syndrome is failure of the respiratory epithe-lium to secrete adequate quantities ofsurfactant, a sub-stance normally secreted into the alveoli that decreases the surface tension of the alveolar fluid, therefore allow-ing the alveoli to open easily during inspiration. The sur-factant-secreting cells (type II alveolar epithelial cells) do not begin to secrete surfactant until the last 1 to 3 months of gestation. Therefore, many premature babies and a few full-term babies are born without the capa-bility to secrete sufficient surfactant, which causes both a collapse tendency of the alveoli and development of pulmonary edema.
Circulatory Readjustments at Birth
Equally as essential as the onset of breathing at birth are immediate circulatory adjustments that allow ade-quate blood flow through the lungs. Also, circulatory adjustments during the first few hours of life cause more and more blood flow through the baby’s liver, which up to this point has had very little blood flow. To describe these readjustments, we must first consider the anatom-ical structure of the fetal circulation.
Specific Anatomical Structure of the Fetal Circulation. Becausethe lungs are mainly nonfunctional during fetal life and because the liver is only partially functional, it is not necessary for the fetal heart to pump much blood through either the lungs or the liver. However, the fetal heart must pump large quantities of blood through the placenta. Therefore, special anatomical arrangements cause the fetal circulatory system to operate much differently from that of the newborn baby.
First, as shown in Figure 83–4, blood returning from the placenta through the umbilical vein passes through the ductus venosus, mainly bypassing the liver. Then most of the blood entering the right atrium from the inferior vena cava is directed in a straight pathway across the posterior aspect of the right atrium and through the foramen ovale directly into the left atrium. Thus, the well-oxygenated blood from the placenta enters mainly the left side of the heart, rather than the right side, and is pumped by the left ventricle mainly into the arteries of the head and forelimbs.
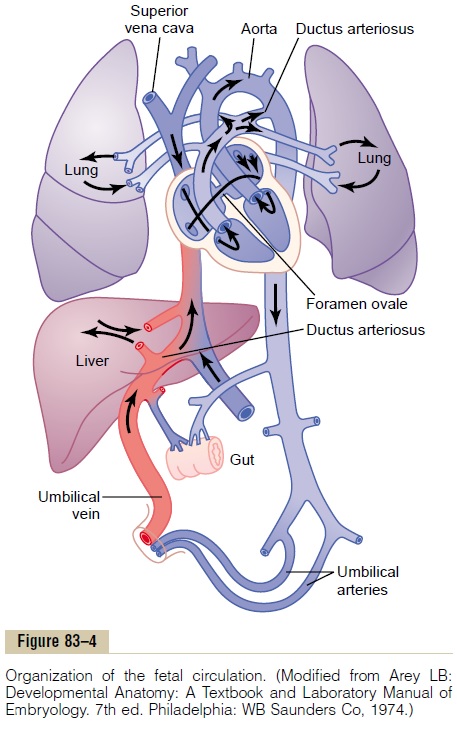
The blood entering the right atrium from the supe-rior vena cava is directed downward through the tri-cuspid valve into the right ventricle. This blood is mainly deoxygenated blood from the head region of the fetus, and it is pumped by the right ventricle into the pul-monary artery and then mainly through the ductus arte-riosus into the descending aorta, then through the twoumbilical arteries into the placenta, where the deoxy-genated blood becomes oxygenated.
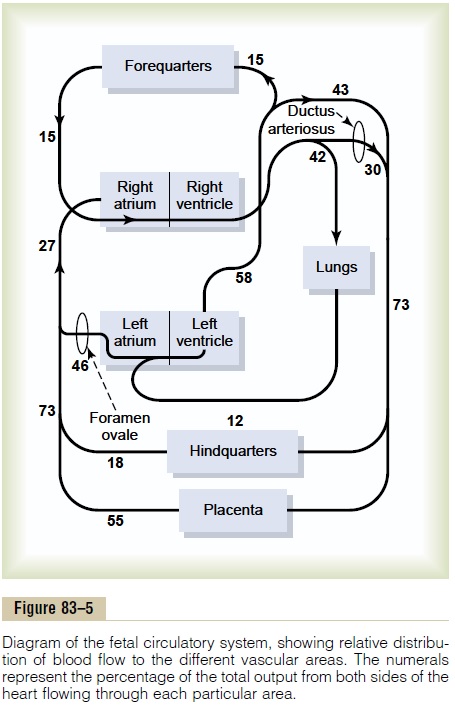
Figure 83–5 gives the relative percentages of the total blood pumped by the heart that pass through the dif-ferent vascular circuits of the fetus. This figure shows that 55 per cent of all the blood goes through the pla-centa, leaving only 45 per cent to pass through all the tissues of the fetus. Furthermore, during fetal life, only 12 per cent of the blood flows through the lungs; imme-diately after birth, virtually all the blood flows through the lungs.
Changes in the Fetal Circulation at Birth. The basic changesin the fetal circulation at birth are discussed in relation to congenital anomalies of the ductus arteriosus and foramen ovale that persist throughout life in a few persons. Briefly, these changes are the following.
Primary Changes in Pulmonary and Systemic Vascular Resistances at Birth
The primary changes in the circulation at birth are, first, loss of the tremendous blood flow through the placenta, which approximately doubles the systemic vascular resistance at birth. This increases the aortic pressure as well as the pressures in the left ventricle and left atrium.
Second, the pulmonary vascular resistance greatlydecreases as a result of expansion of the lungs. In theunexpanded fetal lungs, the blood vessels are com-pressed because of the small volume of the lungs. Imme-diately on expansion, these vessels are no longer compressed and the resistance to blood flow decreases severalfold. Also, in fetal life, the hypoxia of the lungs causes considerable tonic vasoconstriction of the lung blood vessels, but vasodilation takes place when aera-tion of the lungs eliminates the hypoxia. All these changes together reduce the resistance to blood flow through the lungs as much as fivefold, which reduces thepulmonary arterial pressure, right ventricular pressure,and right atrial pressure.
Closure of the Foramen Ovale
The low right atrial pressure and the high left atrial pres-sure that occur secondarily to the changes in pulmonary and systemic resistances at birth cause blood now to attempt to flow backward through the foramen ovale; that is, from the left atrium into the right atrium, rather than in the other direction, as occurred during fetal life. Consequently, the small valve that lies over the foramen ovale on the left side of the atrial septum closes over this opening, thereby preventing further flow through the foramen ovale.
In two thirds of all people, the valve becomes adher-ent over the foramen ovale within a few months to a few years and forms a permanent closure. But even if per-manent closure does not occur, the left atrial pressure throughout life normally remains 2 to 4 mm Hg greater than the right atrial pressure, and the backpressure keeps the valve closed.
Closure of the Ductus Arteriosus
The ductus arteriosus also closes, but for different reasons. First, the increased systemic resistance elevatesthe aortic pressure while the decreased pulmonaryresistance reduces the pulmonary arterial pressure. As a consequence, after birth, blood begins to flow backward from the aorta into the pulmonary artery through the ductus arteriosus, rather than in the other direction as in fetal life. However, after only a few hours, the muscle wall of the ductus arteriosus constricts markedly, and within 1 to 8 days, the constriction is usually sufficient to stop all blood flow. This is called functional closure of the ductus arteriosus. Then, during the next 1 to 4 months, the ductus arteriosus ordinarily becomes anatomically occluded by growth of fibrous tissue into its lumen.
The cause of ductus arteriosus closure relates to the increased oxygenation of the blood flowing through the ductus. In fetal life the PO2 of the ductus blood is only 15 to 20 mm Hg, but it increases to about 100 mm Hg within a few hours after birth. Furthermore, many experiments have shown that the degree of contraction of the smooth muscle in the ductus wall is highly related to this availability of oxygen.
In one of several thousand infants, the ductus fails to close, resulting in a patent ductus arteriosus. The failure of closure has been postulated to result from excessive ductus dilation caused by vasodilating prostaglandins in the ductus wall. In fact, administration of the drug indomethacin, which blocks synthesis of prostaglandins, often leads to closure.
Closure of the Ductus Venosus In fetal life, the portal bloodfrom the fetus’s abdomen joins the blood from the umbilical vein, and these together pass by way of the ductus venosus directly into the vena cava immediatelybelow the heart but above the liver, thus bypassing the liver.
Immediately after birth, blood flow through the umbilical vein ceases, but most of the portal blood still flows through the ductus venosus, with only a small amount passing through the channels of the liver. However, within 1 to 3 hours the muscle wall of the ductus venosus contracts strongly and closes this avenue of flow. As a consequence, the portal venous pressure rises from near 0 to 6 to 10 mm Hg, which is enough to force portal venous blood flow through the liver sinuses. Although the ductus venosus rarely fails to close, we know almost nothing about what causes the closure.
Nutrition of the Neonate
Before birth, the fetus derives almost all its energy from glucose obtained from the mother’s blood. After birth, the amount of glucose stored in the infant’s body in the form of liver and muscle glycogen is sufficient to supply the infant’s needs for only a few hours. The liver of the neonate is still far from functionally adequate at birth, which prevents significant gluconeogenesis. Therefore, the infant’s blood glucose concentration frequently falls the first day to as low as 30 to 40 mg/dl of plasma, less than one half the normal value. Fortunately, however, appropriate mechanisms are available for the infant to use its stored fats and proteins for metabolism until mother’s milk can be provided 2 to 3 days later.
Special problems are also frequently associated with getting an adequate fluid supply to the neonate because the infant’s rate of body fluid turnover averages seven times that of an adult, and the mother’s milk supply requires several days to develop. Ordinarily, the infant’s weight decreases 5 to 10 per cent and sometimes as much as 20 per cent within the first 2 to 3 days of life. Most of this weight loss is loss of fluid rather than of body solids.
Related Topics