Chapter: Microbiology and Immunology: Complement System
Activation of Complement
Activation of Complement
Complement activation takes place through any of the following
three pathways:
1.
The classical pathway
2.
The alternative pathway
3.
The lectin pathway
Of these, alternative and lectin pathways are important in the
innate immunity of the host. These two are also more impor-tant when the human
host is infected by a microorganism for the first time, because the antibody
required to trigger the clas-sical pathway is not present.
All the three activation pathways lead to activation of C3,
resulting in the production of C3b. Hence, C3b is consi-dered as the central
molecule in the activation of the complement cascade.
The final steps that lead to
the formation of a membrane attack complex are same in all the pathways. When
these complement components are activated, a sequential, rapid cascading
pattern ensues. This is because once a complement component is activated, it is
either cleaved or becomes bound to a previously activated component or complex
of complement components. Also, each component or complex of components, once
activated, generally amplifies the cascading process by acti-vating many
molecules of the next component in the series.
Classical Pathway of Complement Activation
The classical pathway is a chain of events in which complement
components react in specific sequences as a cascade resulting in cell lysis. It
is activated by antibody bound to antigen but never by native or free antibody.
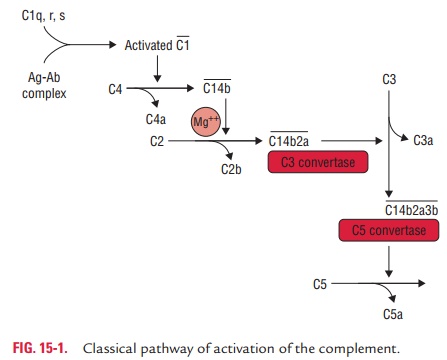
Steps of activation of
classical pathway: The classicalpathway of complement activation usually begins with
the formation of soluble antigen–antibody complexes (immune complexes) or with
the binding of antibody to antigen on a suitable target, such as a bacterial
cell (Fig. 15-1). Following are the sequential steps in the activation of
classical pathway:
1. Activation of C1 is the first step in the cascade of classical pathway activation. The C1 actually is a complex of three different types of molecules: C1q, C1r, and C1s. C1q first combines with the Fc portion of the bound antibody, IgM or IgG. This results in the sequential activation of C4, C2, and C3. For C1 to be activated, it must bind to at least two adjacent Fc regions. This means that the concentration of antibody of the IgG class must be relatively high and that the specific antigenic determinants recognized by the IgG antibody must be in close proximity. When pentameric IgM is bound to antigen on a target surface, it assumes the so-called stable configuration, in which at least three bind-ing sites for the C1q are exposed. Since IgG molecules have a lower valency, about 1000 of them are needed to ensure the initiation of the complement pathway as against only one IgM molecule.
2.
C1q binding in the presence of calcium ions leads to activation of
C1r and C1s. Activated C1s is an esterase that splits C4 into two fragments: a
small soluble frag-ment (C4a) and a larger fragment (C4b). C4a has
ana-phylatoxin activity, and C4b binds to cell membrane along with C1. C4b in
the presence of Mg21 splits C2 into C2a and C2b. The smaller
fragment (C2b) diffuses away, while the larger fragment (C2a) remains attached
to C4b. The resulting C4b2a complex possesses enzymatic activ-ity and is called
C3
convertase, which converts C3 into an active form.
3.
The C3 convertase activate thousands of C3 molecules and splits
these molecules into C3a and C3b. A single C3 convertase molecule can generate
over 200 mol-ecules of C3b, resulting in tremendous amplification at this step
of the sequence. The biological importance of activated C3b as well as C4b is
that they are able to bind to C3b/C4b receptors (currently designated as CR1receptors) present on almost all host
cells, most notablyphagocytes.
The increased affinity of phagocytic cells for C3b (or
iC3b)/C4b-coated particles is known as immune adherence. The latter is
responsible for a significant enhancement of phagocytosis, which is one of the
main defense mecha-nisms of the body.
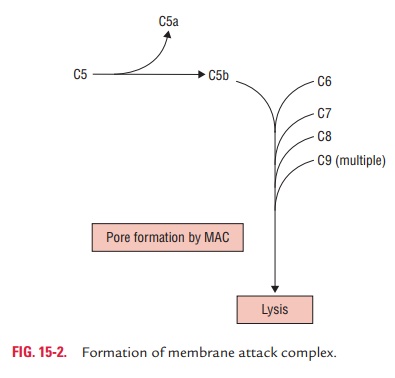
4.
Some of the C3b binds to C4b2a to form a trimolecu-lar complex
C4b2a3b called C5 convertase. The C5 con-vertase splits C5 into C5a and C5b.
C5a diffuses away, while C5b attaches to C6 and initiates formation of C5b–9
complex otherwise known as membrane attackcomplex (MAC).
Released C5b67 complexes can insert into the membrane of nearby
cells and mediate “innocent-bystander” lysis. Regulator proteins in human sera
normally prevent this from occurring, but in certain diseases cell and tissue
damage may occur due to this process of innocent-bystander lysis.
The membrane-bound C5b–6–7 complex acts as a recep-tor for C8 and
C9. C8, on binding to the complex, stabi-lizes the attachment of the complex to
the foreign cell membrane. The C5b–8 complex acts as a catalyst for C9, which
is a single chain glycoprotein with a tendency to polymerize spontaneously.
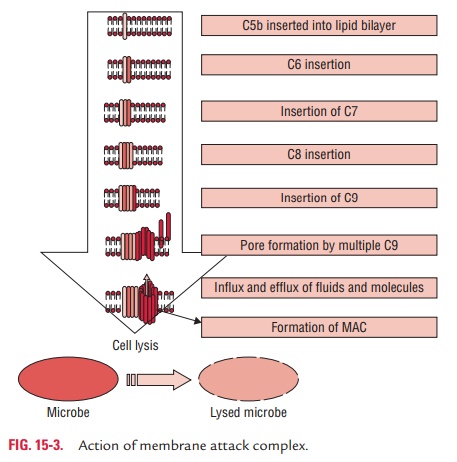
5.
The C5b–8 complex on binding to C9 molecules under-goes
polymerization, which finally ends in the forma-tion of C5b–9 complex also
known as MAC. The MAC forms a transmembrane channel of 100 Å diameter in the
cell. This transmembrane channel allows the free exchange of ions between the
cell and the surrounding medium. Due to the rapid influx of ions into the cell
and their association with cytoplasmic proteins, the osmotic pressure rapidly
increases inside the cell. This results in an influx of water, swelling of the
cell, and, for certain cell types, rupture of the cell membrane and finally
lysis (Fig. 15-3).
Alternative Pathway of Complement Activation
The alternative pathway was first described by Pillemer in 1954. It
differs from the classical pathway in (a)
the nature of activat-ing substances and (b)
the sequence of events itself. The alter-native pathway is unique in not
requiring antigen–antibody complexes to activate the complement. This pathway
does not depend on antibody and does not involve the early complement
components (C1, C2, and C4) for activation of the comple-ment. It, therefore,
can be activated before the establishment of an immune response to the
infecting pathogen (Fig. 15-4).
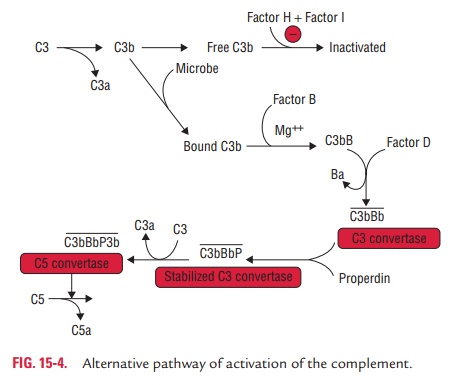
Steps of activation of
alternative pathway: The initial com-ponent of the alternative pathway involves four
serum proteins: C3b, factor B, factor D, and properdin.
1.
The C3b binds with factor B to form C3bB complex. The interaction
between C3b and factor B is stabilized by Mg21, which is the only ion
required for functional activation of the alternative pathway. Therefore, tests
to discriminate between the two complement activation pathways are often based
on the selective chelation of Ca21 (to disrupt C1q, C1r2, and C1s2)
and the addition of sufficient Mg21 to allow activation of the
alternative pathway.
2.
The C3bB is split into two fragments, Ba and Bb, by another serum
protein called factor D or C3 proactive convertase. Since factor D has never
been isolated in its proenzyme form, it is generally believed to be activated
immediately upon leaving the hepatocyte where it is synthesized. The Ba is
released into the medium and the Bb binds to C3b form-ing the C3bBb complex,
which possesses the C3 convertase activity.
3.
The C3bBb complex activates more C3, leading to the for-mation of
more C3bBb, which in turn is capable of activat-ing C5 and the MAC. The C3bBb
complex has a half-life of only 5 minutes, but by binding with properdin it
forms PC3bBb complex, which is relatively heat stable.
4.
The alternative pathway then proceeds from C3 to produce finally
the MAC, in the same way as occurs in the classical pathway.
Lectin Pathway of Complement Activation
The lectin pathway, as the name suggests, is triggered by lec-tins.
Lectins are the proteins that recognize and bind to spe-cific carbohydrate
targets. The mannose-binding lectin (MBL) is one such protein that takes part
in the lectin pathway of complement activation. MBL is a large serum protein
that binds to nonreduced mannose, fructose, and glucosamine on bacterial and
other cell surfaces with mannose-containing polysaccharides (mannans) (Fig. 15-5).
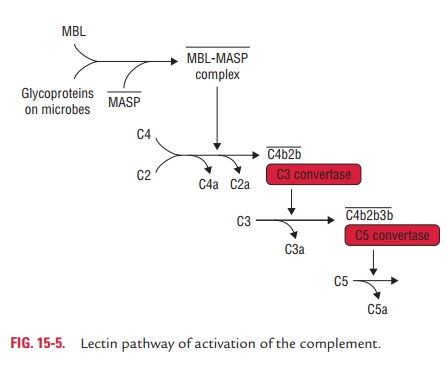
The binding of MBL to a pathogen results in the secretion of two
MBL-associated serine proteases: MASP-1 and MASP-2. MASP-1 and MASP-2 are
similar to C1r and C1s, respectively, and MBL is similar to C1q. Formation of
the MBL/MASP-1/ MASP-2 trimolecular complex results in activation of MASPs and
subsequent cleavage of C4 into C4a and C4b. Subsequently, it proceeds to
produce MAC in the same way as that occurs in the classical and alternative
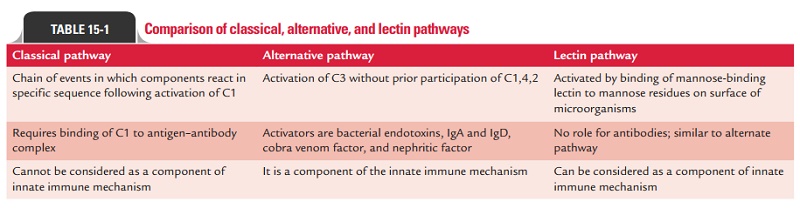
pathways. Differences between classical, alternative, and lectin pathways are
summarized in Table 15-1.
Related Topics