Chapter: Basic Concept of Biotechnology : Antibiotics: Microbial Sources, Production and Optimization
Actinomycetes
Actinomycetes
The phylum actinomycetes are one of the largest groups in the domain bacteria largely consists of environmental bacteria and the denizens of many varied habitat soils such as the rhizosphere, marine and extreme arid environments. Actinomycetes typically have elevated guanosine-cytosine contents (65-75% G + C) and their genome sizes range from 2.5-Mb skin commensal Micrococcus luteus to the 9.7-Mb environmental strainRhodococcusjostii. Since the discovery of antibiotics in the 1940s, the actinomycetes have received a great deal of attention, and Streptomyces species in particular have become renowned as the principal sources of therapeutic pharmaceuticals. There have beenseveral good reviews on actinomycetes of late, notably that by Ventura et al. (2007) on evolutionary and genomic aspects, as well as occasionalarticles focusing on specific genera.
However, other genera, including Rhodococcus, are beginning to excite more interest and Streptomyces may command less attention in the future (Larkin et al., 2005, Kitagawa and Tamura, 2008). Streptomycetes are demonstrably a rich source of compounds, but no more so than other members of the actinomycetes, also the Bacilli and bacteria genera such as the myxobacteria (Wenzel and Muller, 2009) and pseudomonas (Gross and Loper, 2009). Among the eukaryotes, fungal genomes are replete with biosynthetic gene clusters for encoding small molecule production. The ability to make bioactive small molecules is not exclusive to microbes. There is a global crisis in the treatment of infectious diseases and people are dying of infections that were previously treatable. Microbes are the source and the solution for the crisis, and for this reason it is imperative that the search for novel therapeutic agents be intensified. The constant moan of the pharmaceutical industry, that the natural reservoir of molecules with antibiotic activity is close to being exhausted and that they can find no useful bioactive compounds. It can also be explained by the inability to detect bioactive compounds when they are present only in low concentrations. The industry has found all the easily accessible bioactive called ‘‘low hanging fruit’’ (Baltz, 2006). Presumably it was not considered essential to develop the technology to find compounds that were missed.
In addition, actinomycetes as a whole have been ignored in the past years even though they too possess the capacity to produce a huge number of bioactive small molecules. Till date, only a small proportion of actinomycetes have been examined for therapeutic purposes. We are now in the ‘‘genomic era’’ and in the case of Streptomycetes, excitingnew information coming from complete genome sequencing efforts reveals that most of these bacteria have the genetic capacity to produce many more structurally different bioactive compounds than suspected. As such, they represent an inexhaustible collection of hidden chemical and biochemical diversity. Moreover, creative techniques for generating some of these compounds are being developed and exploited (Baltz, 2008; Challis, 2008).
Marine actinomycetes
Actinomycetes represent a ubiquitous group of microbes widely distributed in natural ecosystems around the world and especially significant for their role in the recycling of organic matter (Srinivasan etal., 1991). Actinomycetes are abundant in terrestrial soils, a source ofmost isolates shown to produce bioactive compounds. Goodfellow and Haynes (1984) reviewed the literature on the isolation of actinomycetes from marine sediments and suggested that this source may be valuable for the isolation of novel actinomycetes with the potential to yield useful new products. Earlier studies (Weyland, 1981; Helmke and Weyland, 1984) considered actinomycetes to be part of an indigenous marine microflora. Others saw them primarily as wash in components that nearly survived in marine and littoral sediments as spores (Goodfellow and Williams, 1983). Jensen et al. (1991) and Takizawa et al. (1993) reported a bimodal distribution of actinomycetes in near shore tropical marine environments. The existence of an autochthonous actinomycete population was suggested by the isolation of actinomycetes from marine deep oceanic sediments (Takizawa et al., 1993; Ravel et al., 1998; Weyland and Helmke, 1988).
Although the diversity of life in the terrestrial environment is extraordinary, the greatest biodiversity is in the oceans. More than 70% of our planet’s surface is covered by oceans and life on earth originated from the sea. In some marine ecosystems, such as the deep sea floor andcoral reefs experts estimate that the biological diversity is higher than in the tropical rainforests. As marine environmental conditions are extremely different from terrestrial ones, it is surmised that marine actinomycetes have different characteristics from those of terrestrial counterparts and, therefore, might produce different types of bioactive compounds. The living conditions to which marine actinomycetes had to adapt during evolution range from extremely high pressure and anaerobic conditions at temperatures just below 0˚C on the deep sea floor, to high acidic conditions (pH as low as 2.8) at temperatures of over 10˚C near hydrothermal vents at the mid-ocean ridges. It is likely that this is reflected in the genetic and metabolic diversity of marine actinomycetes, which remains largely unknown. Indeed, the marine environment is a virtually untapped source of novel actinomycetes diversity and, therefore, of new metabolites. However, the distribution of actinomycetes in the sea is largely unexplored and the presence of indigenous marine actinomycetes in the oceans remains elusive. This is partly caused by the lack of effort spent in exploring marine actinomycetes, whereas terrestrial actinomycetes have been, until recently, a successful source of novel bioactive metabolites. Furthermore, skepticism regarding the existence of indigenous populations of marine actinomycetes arises from the fact that the terrestrial bacteria produce resistant spores that are known to be transported from land into sea, where they can remain available but dormant for many years. Thus, it has been frequently assumed that actinomycetes isolated from marine samples are merely of terrestrial origin.
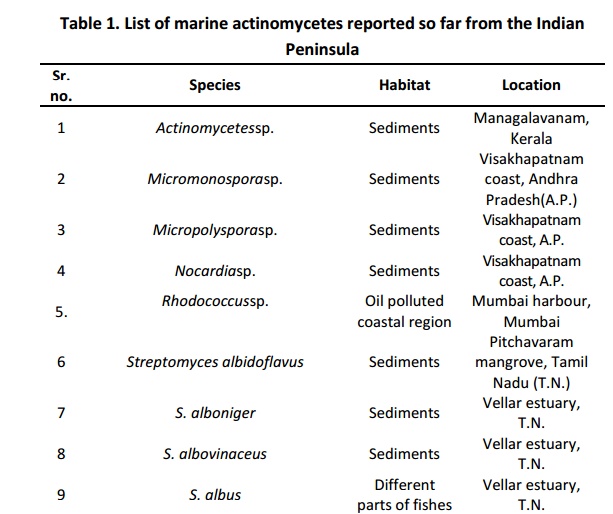
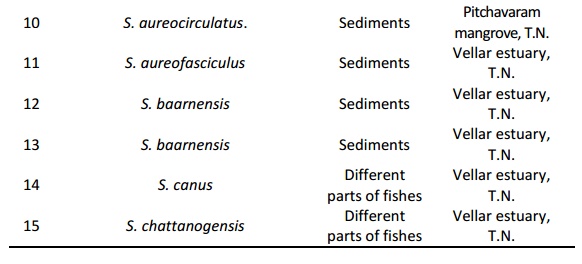

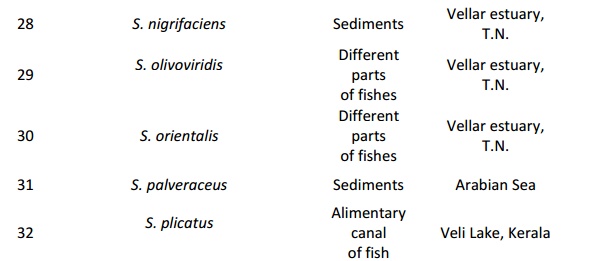
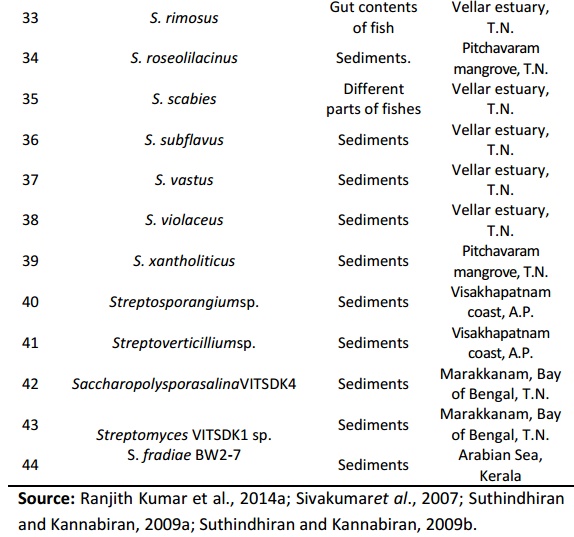
Streptomyces
The search for new antibiotics or new antibiotic producing microbial strains continues to be of utmost importance in research programs around the world because of the increase of resistant pathogens and toxicity of some used chemical antibiotics. Among actinomycetes a large number of antibiotics were obtained from the genus Streptomyces (Alan and James, 2007; Lyudmila et al., 2008; Junker et al., 2009; Koch and Loffler, 2009). Streptomyces are widely recognizedas industrially important microorganisms because of their ability to produce many kinds of novel secondary metabolites including antibiotics (Williams et al., 1983). Indeed, different Streptomyces species produce about 75% of commercially and medically useful antibiotics (Miyadoh, 1993).
Streptomyces species are distributed widely in marine and terrestrial habitats (Pathomareeet al., 2006) and are of commercial interest due to their unique capacity to produce novel metabolites. In fact, the genus Streptomyces alone accounts for a remarkable 80% of the actinomycete natural products reported to date, a biosynthetic capacity that remains without rival in the microbial world (Watveet al., 2001). It was also expected that Streptomyces species will have a cosmopolitan distribution, as they produce abundant spores that are readily dispersed (Antony-Babuet al., 2008). These filamentous bacteria are well adapted to the marine environment and are able to break down complex biological polymers. Marine Streptomyces are widely distributed in biological sources such as fishes, molluscs, sponges, seaweeds, mangroves, besides seawater and sediments. The genus Streptomyces was classified under the family Streptomycetaceae, which includes Gram-positive aerobic members of the order Actinomycetales and suborder Streptomycineae within the new class Actinomycetes (Stackebrandtet al., 1997; Anderson and Wellington, 2001). They have DNA G ± C content of 69–78 mol%. These organisms are gaining importance not only for their taxonomic and ecological perspectives, but also for their production of novel bioactive compounds like antibiotics, enzymes, enzyme inhibitors, pigments and for their biotechnological application such as probiotics and single cell protein.
Related Topics