Chapter: Modern Analytical Chemistry: Introduction
What Is Analytical Chemistry?
What Is Analytical Chemistry?
“Analytical chemistry is what analytical chemists do.”*
We begin this section with a deceptively simple question. What is analytical chem- istry? Like all fields of chemistry, analytical chemistry is too broad and active a disci-
pline for us to easily
or completely. Instead, we will try to say a little about
what analytical chemistry is, as well
as a little about what analytical chemistry is not.
Analytical chemistry is often described as the area of chemistry responsible for
characterizing the composition of matter, both qualitatively (what is present) and quantitatively (how much is present).
This description is misleading. After all, al- most all chemists routinely make qualitative or quantitative measurements. The ar- gument has been made that analytical chemistry is not a separate
branch of chem- istry, but simply the application of chemical knowledge.1 In fact, you probably
have performed quantitative and qualitative analyses
in other chemistry courses. For ex- ample, many introductory courses
in chemistry include
qualitative schemes for identifying inorganic ions and quantitative analyses involving titrations.
Unfortunately, this description ignores the unique perspective that analytical
chemists bring to the study of chemistry. The craft of analytical chemistry
is not in performing a routine
analysis on a routine sample
(which is more
appropriately called chemical analysis), but in improving established methods, extending existing methods to new types of samples, and developing new methods for measuring
chemical phenomena.
Here’s one example
of this distinction between analytical chemistry and chemi- cal analysis.
Mining engineers evaluate
the economic feasibility of extracting an ore
by comparing the cost of removing the
ore with the
value of its
contents. To esti- mate its value they analyze a sample of the ore. The challenge of developing and val-
idating the method providing this information is the analytical chemist’s responsi-
bility. Once developed, the routine,
daily application of the method
becomes the job of the chemical
analyst.
Another
distinction between analytical chemistry and chemical analysis is that analytical chemists work to improve established methods.
For example, sev- eral factors complicate the quantitative analysis of Ni2+ in
ores, including the presence of a complex
heterogeneous mixture of silicates and
oxides, the low
con- centration of Ni2+ in ores,
and the presence
of other metals
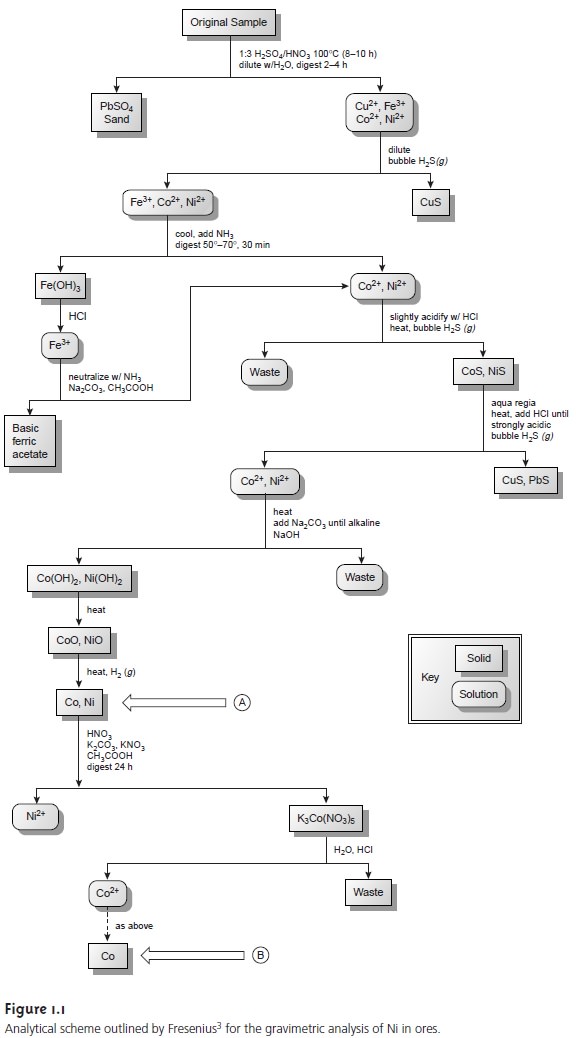
that may interfere in the analysis. Figure
1.1 is a schematic outline
of one standard method in use dur- ing
the late nineteenth century. After
dissolving a sample
of the ore in a mixture
of H2SO4 and HNO3, trace
metals that interfere with the analysis, such as Pb2+, Cu2+ and Fe3+,
are removed by precipitation. Any
cobalt and nickel
in the sample are reduced to Co and Ni, isolated by filtration
and weighed (point A). After dissolving
the mixed solid, Co is isolated
and weighed (point B). The amount of
nickel in the ore sample
is determined from the difference in the masses
at points A and B.
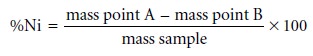
The combination of determining the
mass of Ni2+ by difference, coupled with the need for many reactions and filtrations makes
this procedure both
time-consuming and difficult to perform accurately.
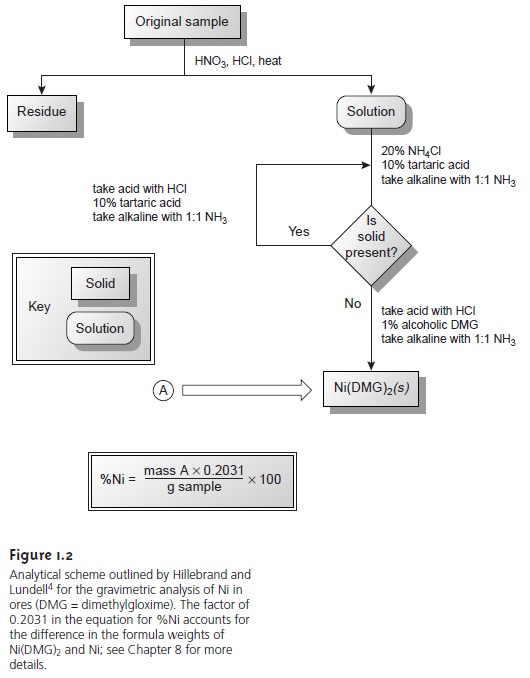
The development, in 1905, of dimethylgloxime (DMG),
a reagent that selec-
tively precipitates Ni2+ and Pd2+,
led to an improved analytical method for deter- mining Ni2+ in ores.4 As shown
in Figure 1.2, the mass of Ni2+ is measured
directly, requiring fewer manipulations and less time.
By the 1970s,
the standard method
for the analysis
of Ni2+ in ores progressed
from precipitating Ni(DMG)2 to flame atomic absorption spectrophotometry,5 resulting in
an even more rapid analysis. Current interest is directed toward
using inductively coupled
plasmas for determin- ing trace metals in ores.
In
summary, a more appropriate description of analytical chemistry is “. . . the science of inventing and applying the concepts, principles, and . . . strategies for measuring the characteristics of chemical systems and species.”6 Analytical
chemists typically operate at the extreme
edges of analysis, extending and improving the abil- ity of all chemists
to make meaningful measurements on smaller samples,
on more complex samples, on shorter time
scales, and on species present
at lower concentra- tions. Throughout its history,
analytical chemistry has provided many of the tools
and methods necessary for research
in the other four traditional areas of chemistry, as well as fostering
multidisciplinary research in, to name a few, medicinal chem- istry, clinical chemistry, toxicology, forensic chemistry, material
science, geochem- istry, and environmental chemistry.
You will come
across numerous examples of qualitative and
quantitative meth- ods in this text,
most of which
are routine examples of chemical analysis. It is im- portant to remember, however,
that nonroutine problems
prompted analytical chemists to develop these
methods. Whenever possible, we will try to place
these methods in their
appropriate historical context. In addition, examples of current re- search problems in analytical chemistry are scattered throughout the text.
The next time
you are in the library, look through a recent issue
of an analyti- cally oriented journal,
such as Analytical Chemistry. Focus on the titles and abstracts
of the research articles. Although
you will not recognize all the terms
and methods, you will begin to answer for yourself the question “What
is analytical chemistry”?
Related Topics