Chapter: The Diversity of Fishes: Biology, Evolution, and Ecology: Living representatives of primitive fishes
Petromyzontiforms - Jawless fishes
Petromyzontiforms
Whereas hagfishes have a bad reputation because of their scavenging habits, many lampreys are parasitic on other vertebrates. Lampreys superfi cially resemble hagfishes in general body form (Fig. 13.4). As with hagfishes, lampreys lack constricted vertebrae, the body being supported by a notochord. They also lack paired fins, jaws, a sympathetic nervous system, and a spleen. They too are scaleless, have a single nostril, and have horny teeth on the tongue. However, adult lampreys possess functional eyes, dorsal fins, an additional semicircular canal, a cerebellum, separate dorsal and ventral roots of the spinal nerves (an innovation among vertebrates), and a spiral-like rather than a straight intestine (Hardisty 1982).
Among the most striking differences between the two jawless fish groups is mode of reproduction. Whereas hagfishes presumably spawn repeatedly during their lives and produce a few large eggs each time, lampreys produce many small eggs and the adults die after spawning. Fecundity
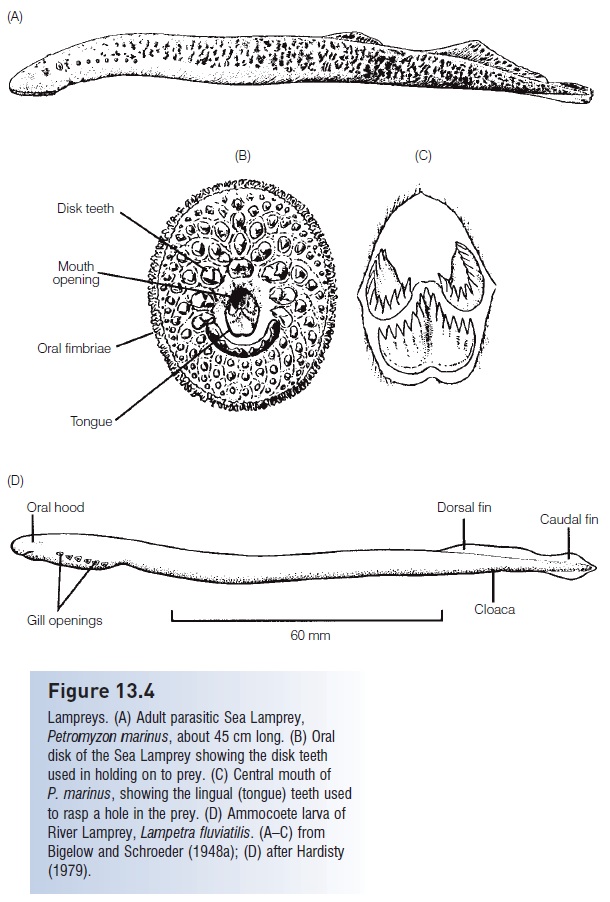
Figure 13.4
Lampreys. (A) Adult parasitic Sea Lamprey, Petromyzon marinus, about 45 cm long. (B) Oral disk of the Sea Lamprey showing the disk teeth used in holding on to prey. (C) Central mouth of P. marinus, showing the lingual (tongue) teeth used to rasp a hole in the prey. (D) Ammocoete larva of River Lamprey, Lampetra fluviatilis. (A–C) from Bigelow and Schroeder (1948a); (D) after Hardisty (1979).
In all lampreys, the free-living, blind, toothless ammocoete typically burrows into the bed of a silty stream or river. Ammocoetes, which were not definitively linked to adult lampreys until the mid-1800s, sit with their heads protruding from the bottom, filtering microscopic organisms from the water column by capturing them on mucus produced in the pharynx. This mode of feeding, possession of an endostyle that later develops into a thyroid gland, and the structure of the pharynx are strongly reminiscent of adult lancelets, supporting hypotheses of relationship between the groups (although lancelets move water through the pharynx via ciliary action, whereas ammocoetes pump water via pharyngeal musculature). Under favorable conditions, ammocoetes can achieve high densities, on the order of 30/m2 (Beamish & Youson 1987). Ammocoetes may live and feed for up to 7 years, achieving a maximum size of about 10–15 cm.
Transformation to the adult stage takes place in summer and fall in most species. In nonparasitic species, called brook or dwarf lampreys, the adult is seldom larger than the ammocoete. After an adult life span of about 6 months, during which no feeding occurs, the adult spawns and dies. In the parasitic species, free-living ammocoetes turn into parasitically feeding adults that may live for 1–3 years before spawning and dying.
Parasitic species are relatively large, up to 120 cm. Parasitic adults attach to the sides of host fishes using the toothed oral disk, rasp a hole in the skin, and live off the blood or body fluids (Ichthyomyzon, Petromyzon, Mordacia) or flesh (Lampetra, Geotria) of their host (Potter & Gill 2003) (Fig. 13.4B,C). Blood loss by the host can be substantial, amounting to 30% of the weight of the lamprey per day. Attachment frequently leads to death of the host. Parasitic lampreys can contribute significantly to the mortality of host species. The River Lamprey, Lampetra ayresi, may kill 18 x106 kg of herring and 10% of the salmon off coastal British Columbia annually (Beamish & Youson 1987).
These natural levels of mortality may have relatively little effect on host population success under normal conditions, but where lampreys have been accidentally introduced, the effects can be catastrophic. The Sea Lamprey, Petromyzon marinus, invaded the upper Laurentian Great Lakes of North America via manmade canals that con- nected the lakes with the Atlantic Ocean. Sea Lampreys have contributed to the decline or extirpation of several fish species, such as Lake Trout, whitefishes, and Blue Pike (Fuller et al. 1999; Daniels 2001). Extensive lampreycontrol strategies, involving chemical larvicides and various methods for trapping adults in spawning tributaries, have apparently helped reduce lamprey populations (Smith 1971; Hanson & Swink 1989; Youson 2003). Ironically, Sea Lampreys are considered imperiled in several European countries because of overfishing, migration blockage from dams, and sedimentation of spawning habitat. In France, among other nations, the species is “highly esteemed for the table” (Keith & Allardi 1996, p. 38).
Anatomical differences between lampreys and hagfishes are strongly reflected in their different foraging tactics. Unlike hagfishes, lampreys have circumferential teeth on the oral disk that aid in grasping live prey (see Fig. 13.4B). The olfactory and respiratory pathways are necessarily separated because lampreys feed and breathe while attached to the exterior of their prey. The nasohypophyseal opening carries water to a blind olfactory organ dorsal to the gill pouches. Attachment involves sealing the contact region between the prey and oral disk via secretion of mucus and the reduction of pressure in the buccal cavity. A vacuum is created as muscles in the mouth and pharyngeal region expel water out of the gill openings. A velar fl ap then seals the branchial chamber off from the buccal and pharyngeal regions, thus maintaining low pressures in the buccal cavity, allowing water to be pumped in and out of the gills for breathing purposes while also keeping food out of the
branchial chamber. Negative pressure in the mouth helps maintain the hold on the prey and also promotes the flow of body fluids from the prey to the lamprey once the rasping tongue has gone to work. Uptake of blood and fluids is further aided by anticoagulants in the lamprey’s saliva (Hardisty 1979).
Blood circulation in the adult lamprey differs from that of the hagfish in several respects. Although lamprey circulation is also “open” in that it is characterized by sinuses connecting arterial and venous systems, these sinuses are not as prevalent as in the hagfish. The primary lamprey sinuses are located in the branchial region and are associated with blood-gas exchange. Lampreys lack the multiple hearts of hagfishes, having instead a large, single, vagally innervated heart in a pericardial cavity, located in the typical fish position posterior to and upstream of the gills. Blood pressure is five times higher in lampreys than in hagfishes.
The reproductive biology of lampreys is well known compared to that of hagfishes. Lampreys also undergo a period of sexual intermediacy when both testicular and ovarian tissues can be found in the developing single gonad, but this period is confined to the ammocoete. During sexual maturation, lampreys undergo radical behavioral, anatomical, and physiological changes that parallel those found in salmon and anguillid eels, other families that die after reproduction (see Death and senescence; Lifetime reproductive opportunities). These changes cause or at least foretell an inability to live beyond the spawning period. Feeding ceases, the gut atrophies, osmoregulatory function shifts, dentition deteriorates, the body shrinks, the eyes and liver degenerate, hematopoiesis (blood production) decreases, and lipid and glycogen stores are reduced. Secondary sex characters, such as thickened fins and genital papillae, are formed.
Upon maturation, adults undertake a spawning migration again reminiscent of the migrations of salmon and eels. Distances moved may range from a few kilometers in nonparasitic or landlocked species to more than 1000 km in anadromous species that move from the ocean to fresh water. Spawning locales are typically the upper regions of streams where bottom types are dominated by gravel and cobbles. These locales are often used in successive years by new generations of lampreys, although little evidence exists to suggest that lampreys return, salmonlike, to spawn in the stream of their birth. Adults on spawning migrations are attracted to suitable areas by detecting a bile acid, petromyzonol sulfate, produced by larvae (Fine et al. 2004). Males then produce a pheromone that is highly attractive to females (Johnson et al. 2006).
The spawning act begins after a male constructs a nest pit by attaching to a rock with the sucker and then carrying the rock downstream or by holding onto large cobbles and thrashing the downstream area. The pit thus created is ringed by large and small cobbles. A female will also take part in nest construction, but site selection appears to be initiated by the male. Nonparasitic species may engage in group spawning in a single nest, whereas larger, parasitic species may engage in pair spawning and male defense of the nest site. When the nest pit is finished, the female attaches to one of the large upstream cobbles, the male attaches to the anterior portion of the female, coils his body around hers, and the two thrash while the male squeezes the eggs out of the female. Spawning occurs repeatedly over 2–9 days and both sexes die within a few days after spawning. Eggs remain in the nest pit for about 2 weeks, and larvae remain in the nest for an additional week. The ammocoetes then drift downstream to areas of slow current and silty or muddy bottoms, where they burrow and begin feeding (Hardisty & Potter 1971; Hardisty 1979).
Lampreys, like hagfishes, are cool water species that seldom occur at latitudes below 30° or in water temperatures above 20°C in either hemisphere. Only two lowlatitude lamprey species are known and both occur at high elevations in Mexico, forming an interesting mirror image to the submergence of tropical species among the entirely marine hagfishes. Nonparasitic forms are entirely confined to fresh water, whereas parasitic forms may occupy fresh water or may be anadromous. Anadromous species hatch in fresh water where they live as larvae, move into coastal
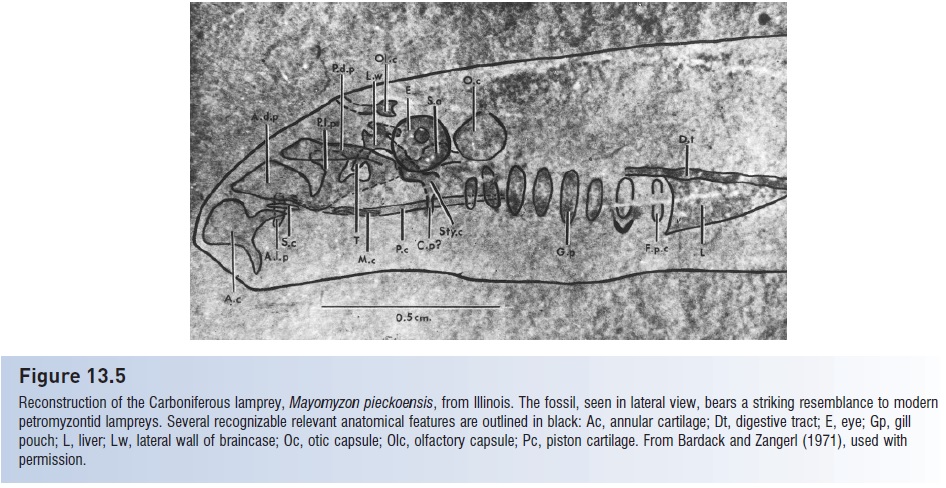
Figure 13.5
Reconstruction of the Carboniferous lamprey, Mayomyzon pieckoensis, from Illinois. The fossil, seen in lateral view, bears a striking resemblance to modern petromyzontid lampreys. Several recognizable relevant anatomical features are outlined in black: Ac, annular cartilage; Dt, digestive tract; E, eye; Gp, gill pouch; L, liver; Lw, lateral wall of braincase; Oc, otic capsule; Olc, olfactory capsule; Pc, piston cartilage. From Bardack and Zangerl (1971), used with permission.
Lamprey taxonomy is based largely on mouth, tentacle, and dentition characteristics (Gill et al. 2003). Lampreys are taxonomically unique in that they have the largest diploid chromosome number of any vertebrate, between 140 and 170 in many northern hemisphere species. The Petromyzontidae of North America and Europe form a monophyletic clade that separated early from the Geotriidae and Mordaciidae of South America, Australia, and New Zealand (Potter & Gill 2003). Two species of an extinct family, the Mayomyzontidae, have been found in 300–320- million-year-old Carboniferous deposits of North America. The more primitive Hardistiella montanensis had a hypocercal tail and lacked an oral sucker, whereas Mayomyzon pieckoensis was relatively small and lacked teeth everywhere except the tongue (Janvier et al. 2004). Mayomyzon is notably similar to modern petromyzontids despite its antiquity (Nelson 1994) (Fig. 13.5).
Paired species in lampreys
Lamprey evolution provides a rare glimpse of ongoing speciation processes: ancestral and derived species exist contemporaneously and often in the same river system (Salewski 2003). At least 18 nonparasitic species, or about half of all lampreys, can be matched to ancestral parasitic forms. The phenomenon has been repeated by 11 species of northern hemisphere, petromyzontid, ancestral species and even one southern hemisphere, mordaciid lamprey (Mordacia mordax gave rise to M. praecox). In some instances, a parasitic river lamprey ancestor has evidently given rise to two or even three nonparasitic brook lamprey species. In each pair, the ammocoetes are almost indistinguishable, except that nonparasitic ammocoetes may grow larger. Although adults of the nonparasitic species are smaller, both species often have the same number of myomeres. Dentition in the adults of the parasitic species is relatively constant in number and shape and in being functionally hooked and sharp. In the nonparasitic, nonfeeding adult species of a pair, dentition is variable and blunt.
Derivation of nonparasitic forms apparently occurred via extension of the larval period and a shortcutting of the metamorphosis process, both intimately linked to the thyroid gland and its hormones (Youson & Sower 2001) (Fig. 13.6). Parasitic species may spend 4 years as larvae and then take 2 years to feed and mature. A corresponding nonparasitic form has a larval period of 6 years, followed by a relatively short, 6 month or less, maturational period. Hybridization between members of a pair is exceedingly rare; this reproductive isolation is maintained largely by size differences. Nonparasitic males, being smaller, cannot coil around and squeeze parasitic females while their vents are in proximity to one another, actions that are necessary for the extrusion of eggs and proper fertilization. Adoption of a shortened, nonparasitic mode of adult existence may expand the range where a species can occur: small brooks may have abundant food resources for larvae but an insuffi cient supply of potential host fishes for a feeding adult.
The phenomenon of repeated, parallel evolution of such paired or “satellite” species is unique among vertebrates (Hardisty & Potter 1971; Vladykov & Kott 1979; Beamish & Neville 1992), and may result from sympatric speciation, where new species arise from old ones without geographic separation (Salewski 2003).
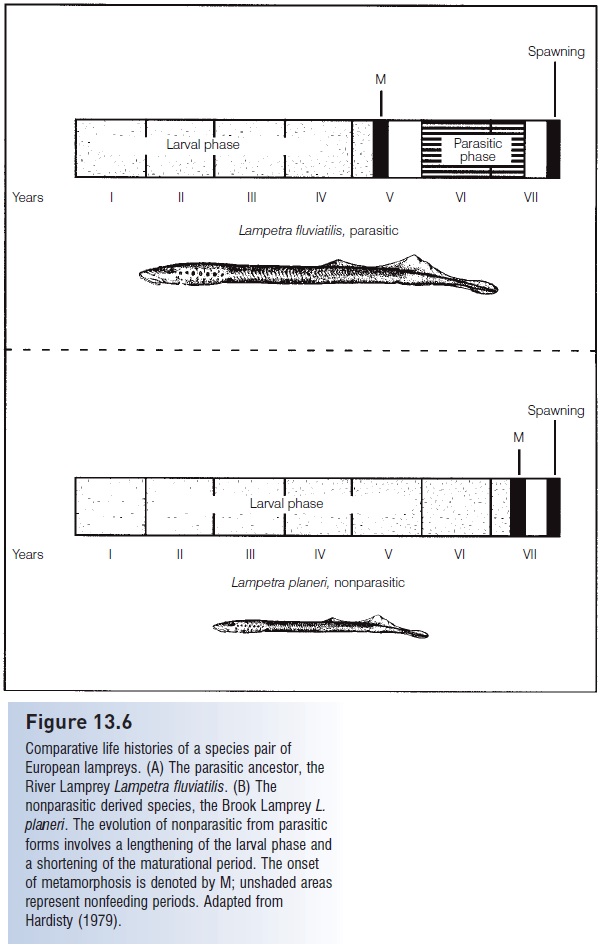
Figure 13.6
Comparative life histories of a species pair of European lampreys. (A) The parasitic ancestor, the River Lamprey Lampetra fluviatilis. (B) The nonparasitic derived species, the Brook Lamprey L. planeri. The evolution of nonparasitic from parasitic forms involves a lengthening of the larval phase and a shortening of the maturational period. The onset of metamorphosis is denoted by M; unshaded areas represent nonfeeding periods. Adapted from Hardisty (1979).
Related Topics