Chapter: The Diversity of Fishes: Biology, Evolution, and Ecology: Living representatives of primitive fishes
Myxiniforms - Jawless fishes
Myxiniforms
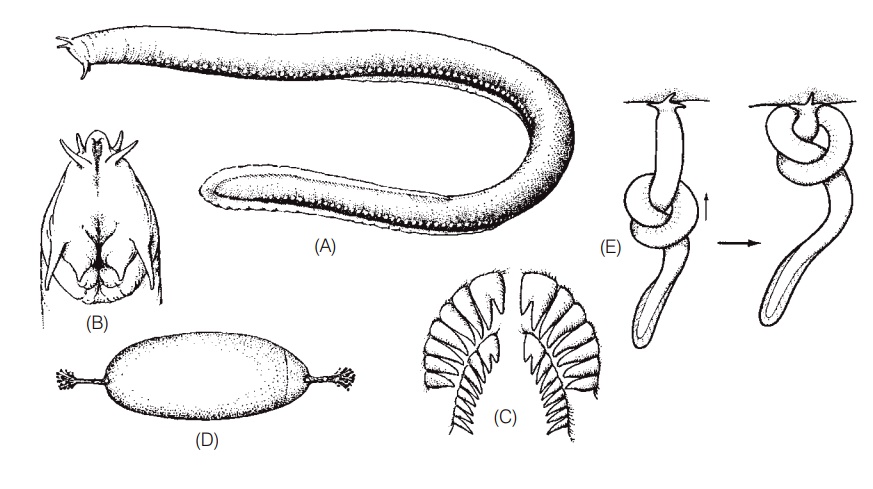
Hagfishes, otherwise known as slime eels or slime hags, derive their alternative names from the copious mucus they produce via 70–200 ventrolateral pairs of slime glands (Fig. 13.2). Mucus production is the combined result of these holocrine slime glands as well as merocrine exudates from the epidermis itself (Spitzer & Koch 1998). The slime glands contain both mucous cells and thread cells, the latter being a unique trait in hagfishes that may strengthen the slime. Each slime gland is surrounded by connective tissue and striated muscle fi bers that help exude the slime upon stimulation. The mucus itself consists of a protein plus a carbohydrate that binds to water and expands to form a loose jelly. A 50 cm hagfish is capable of filling an 8 L bucket with slime in a matter of minutes (Fig. 13.3).
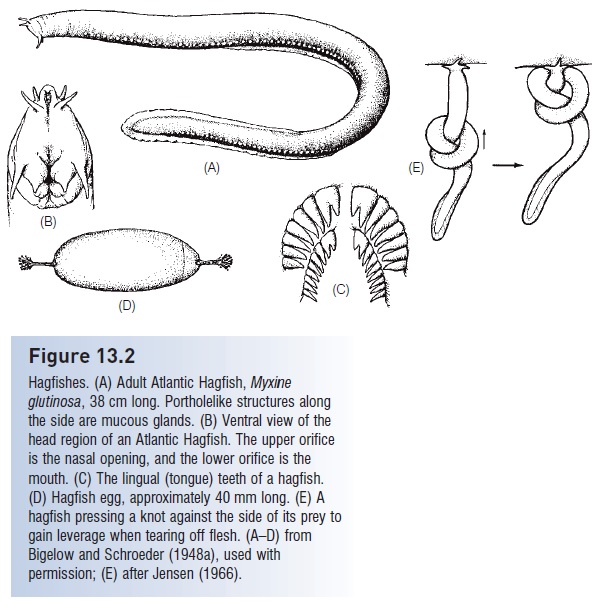
Figure 13.2
Hagfishes. (A) Adult Atlantic Hagfish, Myxine glutinosa, 38 cm long. Portholelike structures along the side are mucous glands. (B) Ventral view of the head region of an Atlantic Hagfish. The upper orifice is the nasal opening, and the lower orifice is the mouth. (C) The lingual (tongue) teeth of a hagfish. (D) Hagfish egg, approximately 40 mm long. (E) A hagfish pressing a knot against the side of its prey to gain leverage when tearing off flesh. (A–D) from Bigelow and Schroeder (1948a), used with permission; (E) after Jensen (1966).
Not surprisingly, slime production may serve multiple functions, some speculative. Authors have suggested: (i) that hagfishes produce slime when attacking dying fish, perhaps hastening suffocation of the prey by clogging its gills; (ii) that mucus could protect the hagfish from digestive enzymes when feeding inside the body of a prey animal; (iii) that mucus is repulsive to other scavengers such as sharks or invertebrates and thus serves to overcome competition; and (iv) that slime stabilizes burrow walls in the muddy bottoms in which hagfishes live (Bigelow & Schroeder 1948a; Brodal & Fange 1963; Hardisty 1979; Smith 1985a). Martini (1998), among the few to actually observe hagfishes in both the field and lab, found no evidence that burrow walls were stabilized with mucus or any other substance.

Figure 13.3
A single hagfish can produce prodigious quantities of slime when disturbed. Photo by J. Meyer
Hagfishes typically produce slime in response to being disturbed or handled. Mucus undoubtedly serves some anti predator function, perhaps by making the fish too slippery to handle or by clogging the gills of a potential predator and threatening it with suffocation. Slime has its drawbacks however. A hagfish covered in its own slime will suffocate after a few minutes. Hagfishes rid themselves of slime by tying an overhand knot in their tail and then sliding the knot forward along the body, pushing the mucus ahead until the knot and mucus reach the anterior end and the fish can back away from the slime mass. A hagfish is also capable of backflushing its gills and nostril with water to rid them of slime (Conniff 1991).
Hagfishes are highly specialized animals belying their typification as “primitive” fishes. They possess four rudimentary hearts: a primary, three-chambered branchial or systemic heart posterior to the gills, and three auxiliary, single-chambered hearts located just behind the mouth (the paired cardinal heart), at midbody (the portal heart), and at the end of the tail (the paired caudal heart). These multiple pumping stations beat at different rates; the branchial and portal hearts contract via intrinsic muscles, whereas the cardinal and caudal hearts are squeezed by surrounding, extrinsic skeletal muscle. The auxiliary hearts are necessary to re-establish blood flow in venous vessels after blood leaves several sinuses where blood flow slows. The sinuses take the place of capillary beds, giving the hagfish a partially open circulatory system, more an invertebrate than a vertebrate trait. Contraction of body wall musculature during activity also aids in pushing blood from the sinuses into adjoining vessels. Hagfish blood is unique among craniates in beingisosmotic with sea water, making it about three times saltier than the blood of bony fishes and lampreys. Hagfish kidneys are much simpler than those of other fishes, including lampreys, and may explain why hagfishes are restricted to a narrow range of salinities (Jensen 1966; Hardisty 1979, 1982; Agnathans).
Other hagfish peculiarities characterize the respiratory, digestive, immune, and sensory systems. Oxygen uptake in hagfishes occurs both at the gills and at capillary beds in the skin. Unlike most fishes, hagfishes inspire water through their nostril and then pump it via the mouth to the gill sacs. Cutaneous respiration comes into play when a hagfish has its nostril and gills buried deep in the carcass of a prey fish. Cutaneous respiration is undoubtedly facilitated by the oxygen-rich nature of the cold waters that hagfishes normally frequent, although the mud in which they bury is often anoxic. As an apparent adaptation to anoxic conditions, mud-burrowing species are exceedingly hypoxia tolerant, able to exist in anoxic conditions for hours or longer (Malte & Lomholt 1998). Low oxygen consumption and very low basal metabolic rates appear to characterize hagfishes. Smith (1985a) calculated that Black Hagfish,Eptatretus deani, could obtain energy suffi cient to maintain itself for 1 year after only 1.5 h of feeding on a high-energy source such as a carcass.
Hagfishes lack a true stomach, having instead an intestine that begins at the pharynx and ends at the anus, with an anterior muscular subdivision that prevents water inflow. Hagfishes do not bleed when their skin is cut, nor do such wounds become infected. Hagfishes have an immune system that produces complement-like factors instead of immunoglobulins (see above); hagfishes lack a defined thymus, spleen, or bone marrow, which are the usual sites of antibody production in vertebrates. Hagfishes also lack complete eyes, but have photosensitive receptors in their head (which contain retinal structures but no lens and are probably incapable of image formation) and cloacal region. Argument over whether the eyeless condition represents a degenerate character or whether the lineage ever possessed true eyes has been solved recently. Fossil material from the Late Carboniferous indicates that Paleozoic hagfishes possessed more developed eyes than recent forms (Bardack 1991; see below). Apparently, visual sensory input has been lost over time in the deep, dark habitats that hagfishes occupy. Food is found largely through olfaction and touch, the six barbels around the mouth serving both functions.
Hagfishes are nocturnal predators on a wide variety of small, benthic invertebrates, but are better known for their scavenging behavior (Shelton 1978; Smith 1990; Martini 1998). Hagfishes have an endearing habit of entering a dead or dying fish or other animal via some orifi ce or by digging through the skin and then consuming their prey from the inside, leaving only the skin and bones and making burial at sea a less-than-appealing proposition. The knot tying action that hagfishes use to deslime their bodies is also employed during feeding. A hagfish grasps a prey item by everting, retracting, and closing its toothplates. It will then pass a knot forward along its body and then press the knot against the prey as a means of levering off a piece of flesh (see Fig. 13.2B). Such knot-feeding is also seen in moray eels. Food is removed via a repeated evert–grasp– retract–release cycle of the toothplates (Martini 1998).
Reproduction in hagfishes remains something of an enigma. Both sexes contain only a single gonad, rather than the paired gonads found in most jawed fishes. In immature animals, this gonad is differentiated anteriorly as ovarian tissue, and posteriorly as testicular tissue. Upon maturation, one cell type prevails and no evidence of functional hermaphroditism has been found (spawning has never been observed). Fertilization is thought to be external since males possess no intromittent organ and females never contain fertilized eggs. Females produce eggs in batches, depositing about 20–30, 1.5–4.0 cm long, heavily yolked, sausageshaped eggs covered by a horny shell (see Fig. 13.2D).
These comparatively large eggs attach to each other and to the ocean floor. Incubation takes about 2 months, development is direct with no larval stage, and the young emerge as 45 mm long replicas of the adults. Most hagfishes show no obvious seasonality in spawning. However, actual spawning times, frequencies, places and behaviors, embryological details, ages at maturity, and reproductive life spans are unknown for most species. A cash prize for information on the reproductive habits of Myxine glutinosa, established in 1854 by the Royal Danish Academy of Sciences, remains unclaimed.
Hagfish species occur almost worldwide in temperate and cold temperate ocean waters above 30° latitude in both hemispheres, although hagfishes are uncommon in polar seas (Hardisty 1979). Few hagfish species occur shallower than 30 m, being limited by both the low salinities and high temperatures found at shallower depths; 34 ppt and 20°C appear to be the minimum salinity and maximum temperatures tolerated (Krejsa et al. 1990a, 1990b). The few tropical species occur in deep water, hagfishes having been captured as deep as 2700 m and photographed at 5000 m. Until recently, hagfishes had little commercial value and were largely viewed as nuisance species that scavenged on more valuable fishes. Hagfishes are preyed upon by dolphins, porpoises, seals, sea lions, and octopus, sometimes accounting for 25–50% of the diet of individual predators (Martini 1998). Human consumption seems to be localized to Asia, where broiled hagfish, called “Anago-yaki” in Japan, is a marketable commodity (e.g., Honma 1998).
Hagfish taxonomy is based on the arrangement of efferent gill ducts (one vs. more excurrent openings), number of slime pores, finfolds, and tentacle and dentition patterns (Fernholm 1998). Some authors recognize two families, the Myxinidae with a single external gill aperture and the Eptatretidae with multiple external gill openings; other workers recognize two subfamilies within the Myxinidae. Maximum lengths range between 25 and 100 cm, with the exception of a recently described giant hagfish from New Zealand that attains a length of at least 127 cm and a mass of 6.2 kg (Mincarone & Stewart 2006). Ongoing analyses indicate several undescribed species in areas where only a single species was thought to occur, although disputes over the validity of some new species exist (e.g., Wisner & McMillan 1990; Nelson 2006).
The only fossil hagfish known is a small, 7 cm long specimen, found in Pennsylvanian (300 million years before present (mybp)) deposits in Illinois (Bardack 1991). This species, Myxinikela siroka – notable for its functional eyes, anteriorly placed gill pouches, and apparent lack of slime pores – is otherwise very similar to extant forms. Its discovery underscores the conservative nature of the hagfish lineage, a clade that may trace its ancestry into Early Paleozoic times via the conodonts (Krejsa et al. 1990a, 1990b).
Related Topics