Chapter: Modern Analytical Chemistry: Kinetic Methods of Analysis
Methods Based on Chemical Kinetics: Instrumentation
Instrumentation
Quantitative information about a chemical
reaction can be made using any of the techniques described in the
preceding. For reactions that are kineti-
cally slow, an analysis may
be performed without
worrying about the
possibility that significant changes in concentration
occur while measuring the signal. When the reaction’s rate
is too fast,
which is usually
the case, significant errors may be introduced if changes in concentration are ignored. One solution to this
problem is to stop, or quench, the reaction
by suitably adjusting experimental conditions. For example, many reactions involving enzymes show a strong pH dependency and may be quenched by adding a strong acid
or strong base.
Once the reaction
is stopped, the concentration of the desired species can be deter- mined at the analyst’s convenience. Another approach is to use
a visual indicator that changes color after
the reaction occurs
to a fixed extent. You may recall
that this variable-time method is the basis of the so-called “clock reactions” com- monly used to demonstrate kinetics in the general chemistry
classroom and labo- ratory. Finally, reactions with fast
kinetics may be monitored continuously using the same types of spectroscopic and electrochemical detectors found in chro- matographic instrumentation.
Two additional
problems for chemical
kinetic methods of analysis
are the need to control
the mixing of the sample and reagents in a rapid and repro- ducible fashion and the
need to control
the acquisition and
analysis of the
signal. Many kinetic determinations are made early
in the reaction when pseudo-zero- order or pseudo-first-order conditions are in effect.
Depending on the rate of re- action, measurements are typically made within a span of a few
milliseconds or seconds. This
is both an advantage and
a disadvantage. The
disadvantage is that transferring
the sample and reagent to
a reaction vessel and their subsequent
mixing must be automated
if a reaction with rapid kinetics is to be practical. This usually requires a dedicated instrument, thereby adding an additional ex- pense to the analysis. The advantage is that a rapid, automated analysis allows for a high throughput of samples. For example, an instrument for the automated ki- netic analysis of
phosphate, based on reaction 13.9, has achieved sampling rates of 3000 determinations per hour.
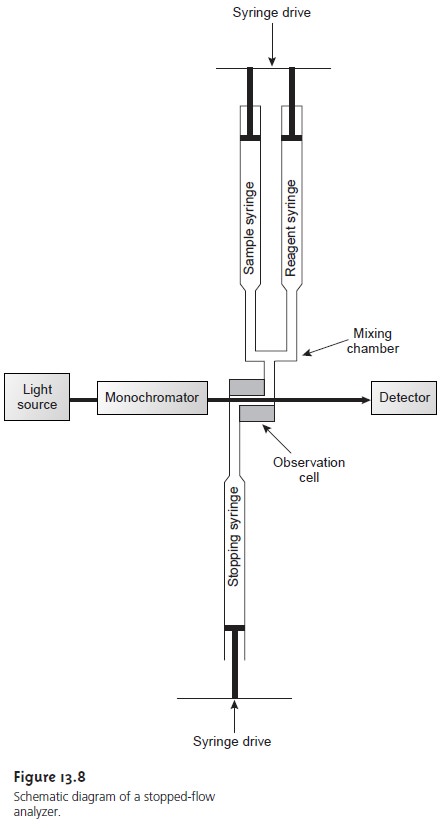
A variety of designs have
been developed to automate kinetic
analyses.6 The
stopped-flow apparatus, which is shown
schematically in Figure
13.8, has found use in kinetic determinations involving very fast
reactions. Sample and
reagents are loaded into separate syringes, and precisely measured
volumes are dispensed by the action of a syringe
drive. The two solutions are rapidly mixed in the mix-
ing chamber before flowing through
an observation cell.
The flow of sample and reagents
is stopped by applying back pressure with the stopping syringe. The back pressure
completes the mixing,
after which the reaction’s progress
is moni- tored spectrophotometrically. With a stopped-flow apparatus, it is possible to complete
the mixing of sample and reagent and initiate the kinetic measure-
ments within approximately 0.5 ms. The stopped-flow apparatus shown in Figure
13.8 can be modified by attaching an automatic sampler
to the sample sy- ringe,
thereby allowing the
sequential analysis of multiple samples. In this way the stopped-flow apparatus can be used for the routine
analysis of several
hun- dred samples per hour.
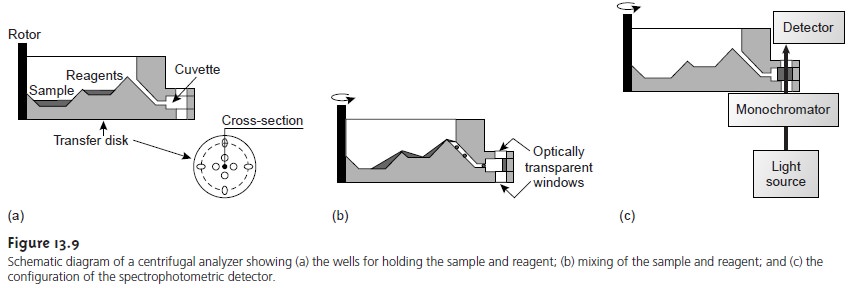
Another automated approach to kinetic analyses is the centrifugal analyzer, a partial cross section of which is shown in Figure 13.9. In this technique
the sample and reagents are placed in separate wells
oriented radially around
a circu- lar transfer disk
attached to the
rotor of a centrifuge. As the centrifuge spins, the sample and reagents are pulled by the centrifugal force to the cuvette, where mixing occurs.
A single optical
source and detector, located above and
below the transfer disk’s outer edge,
allows the absorbance of the reaction
mixture to be measured as it passes through the optical beam. The centrifugal analyzer allows a number of samples to be analyzed simultaneously. For example, if a transfer plate contains 30 cuvettes
and rotates with a speed
of 600 rpm, it is possible to collect 10 data points
per sample for each second
of rotation.
The ability to collect kinetic
data for several
hundred samples per hour is of
little consequence if the analysis
of the data must be accomplished manually. Be- sides time,
the manual analysis
of kinetic data is limited
by noise in the detector’s signal and the accuracy
with which the analyst can determine reaction
rates from tangents drawn
to differential rate
curves. Not surprisingly, the development of automated kinetic analyzers was
paralleled by the development of analog
and digital circuitry, as well as computer software
for the smoothing, on-line integra-
tion and differentiation, and analysis
of kinetic signals.
Related Topics