Chapter: Mechanical : Engineering materials and metallurgy : Heat Treatment Process
Heat Treatment Process
HEAT TREATMENT PROCESS
1 Basic principles of heat treatment
2 Hardening
3 Annealing
4 Normalizing
5 Hardening process
6 Thermo chemical process
7.Tempering
8 Martempering and Austempering
1.BASIC PRINCIPLES OF HEAT
TREATMENT
Heat treatment of a metal or alloy is a
technological procedure, including controlled heating and cooling operations,
conducted for the purpose of changing the alloy microstructure and resulting in
achieving required properties.
There are
two general objectives of heat treatment: hardening and annealing.
2.HARDENING
Hardening is a process of increasing the metal
hardness, strength, toughness, fatigue resistance.
·
Strain hardening (work hardening) – strengthening
by cold work (cold deformation)
Cold
plastic deformation causes increase of concentration of dislocations, which
mutually entangle one another, making further dislocation motion difficult and
therefore resisting the deformation or increasing the metal strength.
·
Grain size strengthening (hardening) -
strengthening by grain refining.
Grain
boundaries serve as barriers to dislocations, raising the stress required to
cause plastic deformation.
• Solid
solution hardening- strengthening by dissolving an alloying element.
Atoms of
solute element distort the crystal lattice, resisting the dislocations motion.
Interstitial elements are more effective in solid solution hardening, than
substitution elements.
•
Dispersion strengthening – strengthening by adding second phase into metal
matrix.
The
second phase boundaries resist the dislocations motions, increasing the
material strength. The strengthening effect may be significant if fine hard
particles are added to a soft ductile matrix (composite materials).
Hardening
by result of Spinodal decomposition. Spinodal structure is characterized by
strains on the coherent boundaries between the Spinodal phases causing
hardening of the alloy.
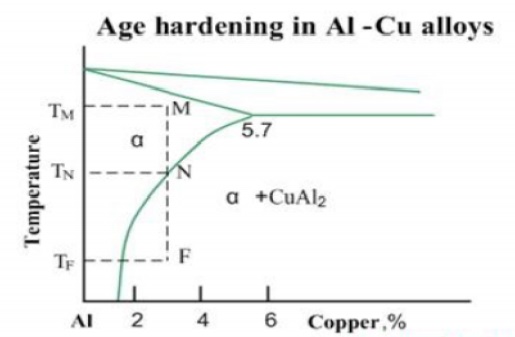
•
Precipitation hardening (age hardening) - strengthening by precipitation of
fine particles of a second phase from a supersaturated solid solution.
The
second phase boundaries resist the dislocations motions, increasing the
material strength. The age hardening mechanism in Al-Cu alloys may be
illustrated by the phase diagram of Al-Cu system. When an alloy Al-3%Cu is
heated up to the temperature TM, all CuAl2 particles are dissolved and the
alloy exists in form of single phase solid solution (α-phase). This operation
is called solution treatment.
Slow
cooling of the alloy will cause formation of relatively coarse particles of
CuAl2 intermetallic phase, starting from the temperature TN.However if the the
cooling rate is high (quenching), solid solution will retain even at room
temperature TF. Solid solution in this non-equilibrium state is called
supersaturated solid solution.
Obtaining
of supersaturated solid solution is possible when cooling is considerably
faster, than diffusion processes. As the diffusion coefficient is strongly
dependent on the the precipitation of CuAl2 from supersaturated temperature,
solution is much faster at elevated temperatures (lower than TN).This process
is called artificial aging. It takes usually a time from several hours to one
day. When the aging is conducted at the room temperature, it is called natural
aging. Natural aging takes several days or more.
Precipitation
from supersaturate d solid solution occurred in several steps:
·
Segregation of Cu atoms into plane clusters. These
clusters are called called Guinier-Preston1 zones (G-P1 zones).
· Diffusion of Cu atoms to the G -P1 zones and
formation larger clusters, called GP2 zones or θ” phase. This phase is coherent
with the matrix .
·
Formation of ‘θ’ phase which is partially coherent
with the matrix. This phase provides maximum hardening.
3.ANNEALING
Annealing
is a heat treatment procedure involving heating the alloy and holding it at a
certain temperature (annealin g temperature), followed by controlled cooling.
Annealing
results in relief of internal stresses, softening, chemical homogenizing and
transformation of the grain structure into more stable state.
Annealing stages:
Stress Relif - a relatively low temperatu re
process of reducing internal mechanical stresses, caused by cold-work, casting
or welding.
During this process atoms move to more stable
positions in the crystal lattice. Vacancies and interstitial defects are
eliminated and some dislocations are annihilate d.
Recovery
heat treatment is used mainly for preventing stress-corrosion cracking and decreasing
distortions, caused by internal stresses.
Recrystallation
-alteration of the grain structure of the metal.
If the
alloy reaches a par ticular temperature (recrystallization or annealing
temperature) new grains start to grow from the nuclei formed in the cold worked
metal. The new grains absorb imperfections an d distortions caused by cold
deformation. The grains are equi-axed and independent to the ol d grain
structure.
As a result of recrystallization mechanical
properties (stre ngth, ductility) of the alloy return to the pre-cold-work
level. The annealing te mperature and the new grains size are dependent on the
degree of cold-wor k which has been conducted. The more the cold-work degree,
the lower the annealing temperature and the fine recrystallization grain
structure. Low d egrees of cold-work (less than 5%) may cause formation of
large grains.Usually the annealing temperature of metals is between one-third
to one-half of t he freezing point measured in Kelvin (absolute) tem perature
scale.
Grains
Growth: (ov er-annealing, secondary recrystallization) - growth of the new
grains at the expense of their neighbors, occurring at temperature, above the
recrystallization temperat ure.
This
process results in coarsening grain structure and is undesirable.
THE SOFTENING PROCESSES
Heat Treatment is the controlled heating and
cooling of metals to alter their physical and mechanical properties without
changing the product shape. Heat treatment is sometimes done inadvertently due
to manufacturing processes that either heat or cool the metal such as welding
or forming. Heat Treatment is often associated with increasing the strength of
material, but it can also be used to alter certain manufacturability objectives
such as improve machining, improve formability, restore ductility after a cold
working operation. Thus it is a very enabling manufacturing process that can
not only help other manufacturing process, but can also improve product
performance by increasing strength or other desirable characteristics. Steels
are particularly suitable for heat treatment, since they respond well to heat
treatment and the commercial use of steels exceeds that of any other material.
Steels are heat treated for one of the following reasons:
Softening
Softening
is done to reduce strength or hardness, remove residual stresses, improve
toughnesss, restore ductility, refine grain size or change the electromagnetic
properties of the steel.
Restoring
ductility or removing residual stresses is a necessary operation when a large
amount of cold working is to be performed, such as in a cold- rolling operation
or wiredrawing.
Annealing
— full Process, spheroidizing, normalizing and tempering— austempering,
martempering are the principal ways by which steel is softened.
Hardening:
Hardening
of steels is done to increase the strength and wear properties. One of the
pre-requisites for hardening is sufficient carbon and alloy content. If there
is sufficient Carbon content then the steel can be directly hardened. Otherwise
the surface of the part has to be Carbon enriched using some diffusion
treatment hardening techniques. Material Modification: Heat treatment is used
to modify properties of materials in addition to hardening and softening. These
processes modify the behavior of the steels in a beneficial manner to maximize
service life, e.g., stress relieving, or strength properties, e.g., cryogenic
treatment, or some other desirable properties, e.g., spring aging.
Annealing
Used
variously to soften, relieve internal stresses, improve machinability and to
develop particular mechanical and physical properties.In special silicon steels
used for transformer laminations annealing develops the particular
microstructure that confers the unique electrical properties.Annealing requires
heating to above the As temperature, holding for sufficient time for
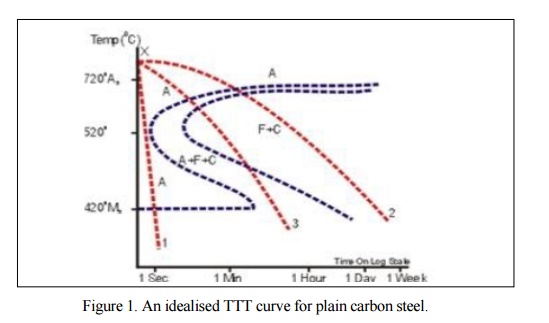
temperature equalisation followed by slow cooling. See Curve 2 in Figure.1
Stress
from the forming operations can affect both rimfire and centerfire cartridge
cases. For many cases, especially those with bottlenecks, the stresses are so
great that high-temperature annealing must be used.After forming, a bottleneck
case may appear perfectly serviceable. However, massive stresses are likely to
remain in these areas. If the ammunition is loaded and stored without
addressing these stresses, cracks can appear in the bottleneck area.

Case bottlenecks are normally flame-annealed by the
following process:
Case
bottlenecks are normally flame-annealed by the following process:
Placed on
a moving rail or rotary disk system, the case passes before a set of gas
burners that rapidly heat the neck and shoulder area to glowing.
As the
becomes incandescent the brass grains grow larger. he heated area of the case
is immediately tipped into a water bath to quench the case, establishing the
large grain size.
The
treatment causes a dark, but harmless, discoloration to the neck area. In
commercial ammunition, this dark area may be polished out for cosmetic reasons;
in U.S. military ammunition, the discoloration remains vi sible.
The
application of heat treatment technology to vary the grain size gradually, from
small grains in the head area to large o nes at the case mouth, determines c
ase hardness. All high pressure cases must have variable metallurgical
properties depending on the part of the case, as follows:
Head - must be t ough and relatively
unyielding, small brass grains contribute to the t oughness.
4.NORMALISING
Also used
to soften and relieve internal stresses after cold work and to refine the grain
size and m etallurgical structure. It may be used t o break up the dendritic
(as cast) str ucture of castings to improve their m achinability and future
heat treatment response or to mitigate banding in rolled steel. This requires
heatin g to above the As temperature, holding for sufficient time to allow tem
perature equalization followed by air co oling. It is therefore similar to
annealing but with a faster cooling rate. Curve 3 in Figure I would give a
normalized structure.
5.THE HARDENING PRO CESSES
Hardening
In this
process steels which contain sufficient carbon, and perhaps other alloying
elements, are cooled (quenc hed) sufficiently rapidly from above the
transformation temperature to produce Martensite, the hard phase already
described, s ee Curve 1 in Figure 1.There is a range of quenching media of
varying severity, water o r brine being the most severe, through oil and
synthetic products to air which is the least severe.
Tempering
After
quenching the steel is hard, brittle and internally stre ssed. Before use, it
is usually necessar y to reduce these stresses and increa se toughness by
'tempering'. There will als o be a reduction in hardness and the selection of
tempering temperature dictates The final properties. Tempering curves, which
are plots of hardness against tempering temperature. exist for all commercial
steels and are used to select the correct tempering temperature. As a rule of
thumb, within the tempering range for a particular steel, the higher the
tempering temperature the lower the final hardness but the greater the
toughness. It should be noted that not all steels will respond to all heat
treatment processes, Table 1 summaries the response, or otherwise, to the
different processes.

Boronised
substrates will often require heat treatment to restore mechanical properties.
As borides degrade in atmospheres which contain oxygen, even when combined as
CO or C02, they must be heat treated in vacuum, nitrogen or nitrogen/hydrogen
atmospheres.
PROCESSING METHODS
In the past the thermochemical processes were
carried out by pack cementation or salt bath processes. These are now largely
replaced, on product quality and environmental grounds, by gas and plasma
techniques. The exception is boronising, for which a safe production scale
gaseous route has yet to be developed and pack cementation is likely to remain
the only viable route for the for some time to come.
The gas processes are usually carried out in the
now almost universal seal quench furnace, and any subsequent heat treatment is
readily carried out immediately without taking the work out of the furnace.
This reduced handling is a cost and quality benefit.
![]()
![]()
![]()
![]()
![]()
![]()
![]()
TECHNIQUES AND PRACTICE
As we
have already seen this requires heating to above the As temperature, holding to
equalise the temperature and then slow cooling. If this is done in air there is
a real risk of damage to the part by decarburisation and of course oxidation.
It is increasingly common to avoid this by ‗bright„ or ‗close„ annealing using
protective atmospheres. The particular atmosphere chosen will depend upon the
type of steel.
NORMALISING
In common
with annealing there is a risk of surface degradation but as air cooling is
common practice this process is most often used as an intermediate stage to be
followed by machining, acid pickling or cold working to restore surface
integrity.
HARDENING
With many
components, hardening is virtually the final process and great care must taken
to protect the surface from degradation and decarburisation. The ‗seal quench„
furnace is now an industry standard tool for carbon, low and medium alloy
steels. The work is protected at each stage by a specially generated
atmosphere.
Some tool
steels benefit from vacuum hardening and tempering; salt baths were widely used
but are now losing favour on environmental grounds.
7.TEMPERING
Tempering
is essential after most hardening operations to restore some toughness to the
structure. It is frequently performed as an integral part of the cycle in a
seal quench furnace, with the parts fully protected against oxidation and
decarburisation throughout the process. Generally tempering is conducted in the
temperature range 150 to 700°C, depending on the type of steel and is time
dependent as the microstructural changes occur relatively slowly.
Caution:
Tempering can,in some circumstances, m a k e the steel brittle which is the
opposite of what it is intended to achieve.
There are
two forms of this brittleness Temper
Brittleness which affects both carbon and low alloy steels when either,
they are cooled too slowly from above 575°C, or are held for excessive times in
the range 375 to 575°C. The embrittlement can be reversed by heating to above
575°C and rapidly cooling.
Blue Brittleness affects carbon and some alloy
steels after tempering in the range 230 to 370°C The effect is not reversible
and susceptible steels should not be employed in applications in which they
sustain shock loads. If there is any doubt consult with the heat treater or in
house metallurgical department about the suitability of the steel type and the
necessary heat treatment for any application.
8.MARTEMPERING AND AUSTEMPERING
It will
be readily appreciated that the quenching operation used in hardening
introduces internal stresses into the steel. These can be sufficiently large to
distort or even crack the steel.
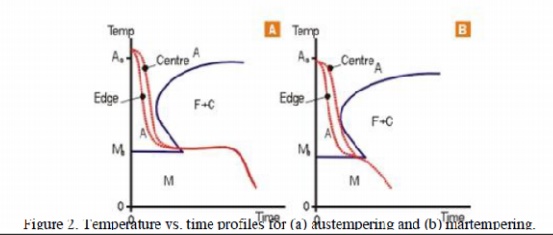
Martempering
is applied to steels of sufficient hardenability and involves an isothermal
hold in the quenching operation. This allows temperature equalisation across
the section of the part and more uniform cooling and structure, hence lower
stresses. The steel can then be tempered in the usual way.
Austempering
also invo lves an isothermal hold in the quenching operation, but the structure
formed, whilst hard and tough, does not require further tempering. The process
is mostly applied to high carbon steels in relatively thin sections for springs
o r similarparts . These processes are shown schematically in the TTT Curves,
(figures 2a and 2b). there is sufficient heat sink in the part and an external
quench is not needed. There is a much lower risk of distortion associated with
this practice, and it can be highly automated and it is very reproducible
Body -
the case walls must combine flexibility and strength to contribute to the
obturati on system.
Mouth -
must be softer (larger brass grains) to prevent cracks from the strain of
holding a bullet.
Related Topics