Chapter: Mechanical : Engineering materials and metallurgy : Constitution of Alloys and Phase Diagrams
Constitution of Alloys and Phase Diagrams
CONSTITUTION OF ALLOYS AND PHASE
DIAGRAMS
1 Classification of materials
2 Type of bonding
3 Crystal structures
4 Imperfection in solids
5 Introduction to phase diagram
6 Solid solution
7 Iron Carbon Diagram
8 Metal types
8.1 Ferrous metals
8.2 Alloy steels
8.3 Non ferrous metals
1 CLASSIFICATION OF MATERIALS
1Metals
Valence electrons are detached from
atoms, and spread in an 'electron
sea' that "glues" the ions together. Metals are usually strong,
conduct electricity and heat well and are opaque to light (shiny if polished).
Examples: aluminum, steel, brass, gold.
2 Semiconductors
The bonding is covalent (electrons are shared
between atoms). Their electrical properties depend extremely strongly on minute
proportions of contaminants. They are opaque to visible light but transparent
to the infrared. Examples: Si, Ge, GaAs.
3Ceramics
Atoms behave mostly like either positive or
negative ions, and are bound by Coulomb forces between them. They are usually
combinations of metals or semiconductors with oxygen, nitrogen or carbon
(oxides, nitrides, and carbides). Examples: glass, porcelain, many minerals.
4.Polymers
Are bound
by covalent forces and also by weak van
der Waals forces, and usually based on H, C and other non-metallic elements. They
decompose at moderate
temperatures (100 - 400 C), and are lightweight. Other
properties vary greatly. Examples: plastics (nylon, Teflon, polyester)
and rubber.
2 TYPES OF BONDING
1 Ionic Bonding
This is the bond when one of the atoms is negative
(has an extra electron) and another is positive (has lost an electron). Then
there is a strong, direct Coulomb attraction. An example is NaCl. In the
molecule, there are more electrons around Cl, forming Cl- and less
around Na, forming Na+. Ionic bonds are the strongest bonds.
1.2.2 Covalent
Bonding
In
covalent bonding, electrons are shared between the molecules, to saturate the valency. The
simplest example is the H2
molecule, where the electrons spend
more time in between the nuclei than outside, thus producing bonding.
3 Metallic Bonding
In the
metallic bond encountered in pure metals and metallic alloys, the atoms
contribute their outer-shell electrons to a generally shared electron cloud for
the whole block of metal.
Ø Secondary
Bonding (Van der Waals)
Ø Fluctuating
Induced Dipole Bonds Polar
Ø Molecule-Induced
Dipole Bonds
Ø Permanent
Dipole Bonds
3 CRYSTAL STRUCTURES
Atoms
self-organize in crystals, most of
the time. The crystalline lattice is a periodic array of the atoms. When the
solid is not crystalline, it is called amorphous. Examples of crystalline
solids are metals, diamond and other precious stones, ice, graphite. Examples
of amorphous solids are glass, amorphous carbon (a-C), amorphous Si, most
plastics
1.Unit Cells
The unit
cell is the smallest structure
that repeats itself by
translation through the crystal. The most common types of unit cells are
the faced centered cubic (FCC), the body-centered cubic (FCC) and the hexagonal
close-packed (HCP). Other types exist, particularly among minerals.
2.Polymorphism and Allotropy
Some
materials may exist in more than one crystal structure, this is called
polymorphism.
If the
material is an elemental solid, it is called allotropy. An example of allotropy
is carbon, which can exist as diamond, graphite, and amorphous carbon.
3.Polycrystalline Materials
A solid
can be composed of many crystalline grains, not aligned with each other. It is
called polycrystalline. The grains
can be more or less aligned with respect to each other. Where they meet is called a grain boundary.
4 IMPERFECTION IN SOLIDS
Materials
are not stronger when they have defects.
The study
of defects is divided according to their dimension:
0D (zero
dimension) - point defects: vacancies and interstitials Impurities. 1D - linear
defects: dislocations (edge, screw, mixed)
2D -
grain boundaries, surfaces. 3D - extended defects: pores, cracks
5 Introduction to phase diagram
Component
Pure
metal or compound (e.g., Cu, Zn in Cu-Zn alloy, sugar, water, in syrup.)
Solvent
Host or
major component in solution.
Solute
Dissolved,
minor component in solution.
System
Set of
possible alloys from same component (e.g., iron-carbon system.)
Solubility Limit
Maximum solute concentration that can be dissolved
at a given temperature.
Phase
Part with
homogeneous physical and chemical characteristics
6 Solid Solutions
A solid solution occurs when we alloy two metals
and they are completely soluble in each other. If a solid solution alloy is
viewed under a microscope only one type of crystal can be seen just like a pure
metal. Solid solution alloys have similar properties to pure metals but with
greater strength but are not as good as electrical conductors.
The common types of solid
solutions are
1) Substitutional
solid solution
2) Interstitial
solid solutions
Substitution solid solution
The name of this solid solution tells you exactly
what happens as atoms of the parent metal ( or solvent metal) are replaced or
substituted by atoms of the alloying metal (solute metal) In this case, the
atoms of the two metals in the alloy, are of similar size.
Interstitial solid solutions:
In interstitial solid solutions the atoms of the
parent or solvent metal are bigger than the atoms of the alloying or solute
metal. In this case, the smaller atoms fit into interstices i.e spaces between
the larger atoms.
Phases
One-phase systems are homogeneous. Systems with two
or more phases are heterogeneous, or mixtures. This is the case of most
metallic alloys, but also happens in ceramics and polymers.
A
two-component alloy is called binary. One with three components is called
ternary.
Microstructure
The properties of an alloy do not depend only on
concentration of the phases but how they are arranged structurally at the
microscopy level. Thus, the microstructure is specified by the number of
phases, their proportions, and their arrangement in space.
A binary
alloy may be
ØA single solid solution
ØTwo separated essentially pure
components. ØTwo separated solid solutions.
ØA chemical compound, together
with a solid solution.
Phase diagram:
A graph showing the phase or phases present for a
given composition as a function of temperature.
Poly phase material:
A
material in which two or more phases are present.
Eutectoid:
Transforming
from a solid phase to two other solid phases upon cooling.
Peritectoid:
Transforming
from two solid phases to a third solid phase upon cooling.
Peritectoid reaction:
A reaction in which a solid goes to a new solid
plus a liquid on heating, and reverse occurs on cooling.
Iron-Iron Carbon diagram is essential to understand
the basic differences among iron alloys and control of properties.
Iron is allotropic; at room temperature pure iron
exists in the Body Centered Cubic crystal form but on heating transforms to a
Face Centered Cubic crystal. The temperature that this first transformation
takes place is known as a critical point and it occurs at 910 degrees Celsius.
This change in crystal structure is accompanied by shrinkage in volume, sine
the atoms in the face centered crystal are more densely packed together than in
the body centered cubic crystal. At the second critical point the F.C.C crystal
changes back to a B.C.C crystal and this change occurs at 1390 degrees Celsius.
§ Iron
above 1390 degrees is known as delta iron
§ Iron
between 1390 and 910 degrees is known as gamma iron, Iron below 910 degrees is
known as alpha iron.
7 IRON CARBON DIAGRAM
Iron-carbon phase diagram
Iron-carbon
phase diagram describes the iron-carbon system of alloys containing up to 6.67%
of carbon, discloses the phases
compositions and their transformations occurring with the alloys during their cooling or heating. Carbon content 6.67% corresponds to the fixed c omposition of the iron carbide Fe3C.
The
diagram is presented in the picture:
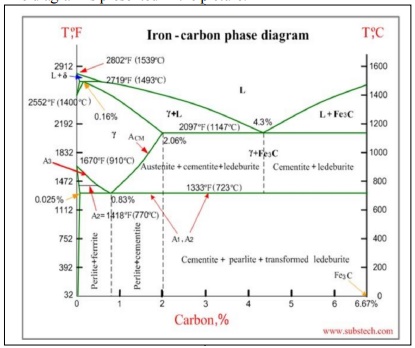
The
following phases are involved in the transformation, occurring with iron-carbon
alloys: L - Liquid solution of carbon in iron;
δ-ferrite - Solid solution of carbon in iron.
Maximum concentration of carbon in δ-ferrite
is 0.09% at 2719 ºF (1493ºC) - temperature of the peritectic transformation.
The crystal structure of δ-ferrite is BCC
(cubic body centered). Austenite - interstitial solid solution of carbon in
γ-iron.
Austenite has FCC (cubic face centered)
crystal structure, permitting high solubility of carbon - up to 2.06% at 2097
ºF (1147 ºC).
Austenite
does not exist below 1333 ºF (723ºC) and maximum carbo n concentration at this
temperature is 0.83%.
α-ferrite
- solid solution o f carbon in α-iron. α-ferrite has BCC crystal structure and
low solubility of carbon - up to 0.25% at 1333 ºF (723ºC). α-ferrite exists at
room temperature.
Cementite
- iron carbide , intermetallic compound, having fixed com position Fe3C.
Cementite
is a hard and brittle substance, influencing on the properties of steels and cast
irons.
The
following phase transformations occur with iron-carbon alloys:
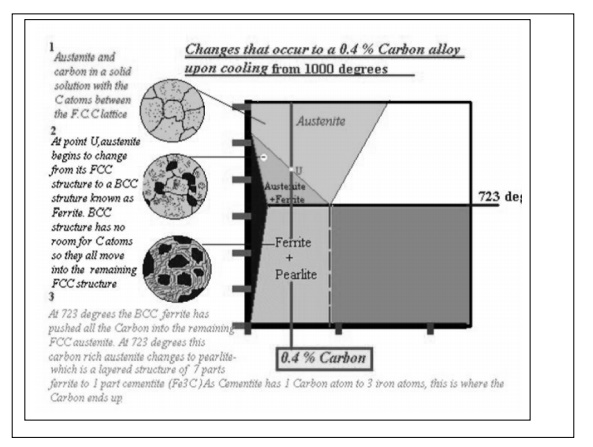
Alloys,
containing up to 0.51% of carbon, start soli dification with formation of
crystals of δ-ferrite. Carbon content in δ-ferrite increases up to 0.09% in
course solidification, and at 2719 ºF (1493ºC) remaining liquid phase and δ-
ferrite perform peritectic transformation, resulting in formation of austenite.
Alloys,
containing carbon more than 0.51%, but less than 2.06%, form primary austenite
crystals in the beginning of solidification and when the temperature reaches
the curve ACM primary cementite stars to form.
Iron-carbon
alloys, containing up to 2.06% of carbon, are called steels.
Alloys,
containing from 2.06 to 6.67% of carbon,
experience eutectic transformation at 2097 ºF (1147 ºC). The eutectic
concentration of carbon is 4.3%.
In
practice only hypoeutectic alloys are used. These alloys (carbon content from
2.06% to 4.3%) are called cast irons. When
temperature of an alloy from
this range reaches 2097 ºF (1147
ºC), it contains
primary austenite crystals and
some amount of
the liquid phase. The latter decomposes by eutectic mechanism to a fine mixture of
austenite and cementite, called ledeburite.
All
iron-carbon alloys (steels and cast irons) experience eutectoid transformation
at 1333 ºF (723ºC). The eutectoid concentration of carbon is 0.83%.
When the
temperature of an alloy reaches 1333 ºF (733ºC), austenite transforms to pearlite
(fine ferrite-cementite structure,
forming as a result
of decomposition of austenite at slow cooling conditions).
Critical temperatures
Upper
critical temperature (point) A3 is the temperature, below which ferrite starts
to form as a result of ejection from austenite in the hypoeutectoid alloys.
Upper
critical temperature (point) ACM is the temperature, below which cementite
starts to form as a result of ejection from austenite in the hypereutectoid
alloys.
Lower
critical temperature (point) A1 is the temperature of the austeniteto-pearlite
eutectoid transformation. Below this temperature austenite does not exist.
Magnetic
transformation temperature A2 is the temperature below which α-ferrite is
ferromagnetic.
Phase
compositions of the iron-carbon alloys at room temperature
o
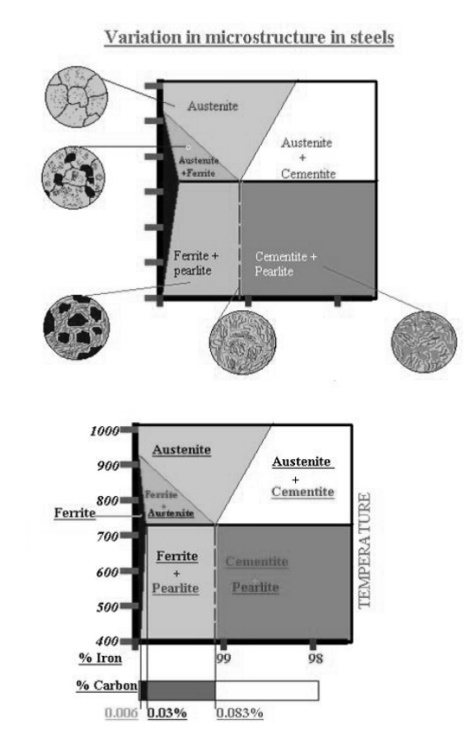
Hypoeutectoid steels (carbon content from 0 to 0.83%) consist of primary
(proeutectoid) ferrite (according to the curve A3) and pearlite.
o
Eutectoid steel (carbon content 0.83%) entirely consists of pearlite.
o
Hypereutectoid steels (carbon content from 0.83 to 2.06%) consist of primary
(proeutectoid) cementite (according to the curve ACM) and pearlite.
o Cast
irons (carbon content from 2.06% to
4.3%) consist of proeutectoid cementite C2 ejected from austenite according to
the curve ACM , pearlite and transformed ledeburite (ledeburite in
which austenite transformed to pearlite).
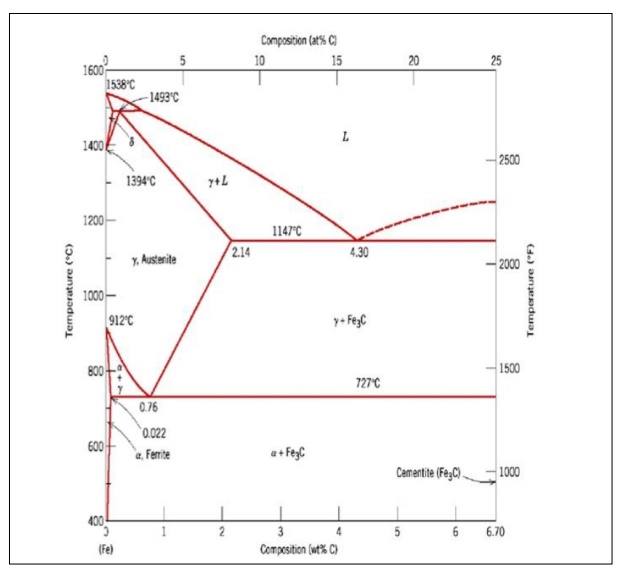
At 4.3%
carbon composition, on cooling Liquid phase is converted in to two solids
hence
forming Eutectic reaction.
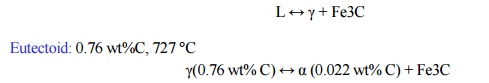
L ↔ γ +
Fe3C
Eutectoid: 0.76 wt%C, 727 °C
γ(0.76
wt% C) ↔ α (0.022 wt% C) + Fe3C
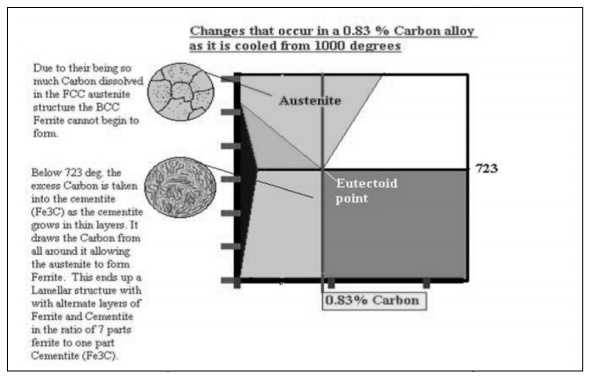
Shown
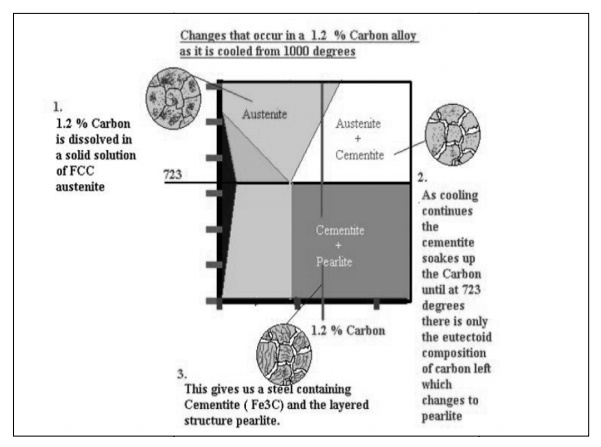
below is the steel part of the iron carbon diagram containing up to 2% Carbon.
At the eutectoid point 0.83% Carbon, Austenite which is in a solid solution
changes directly into a solid known as Pearlite which is a layered structure
consisting of layers of Ferrite and Cementite
8. METAL TYPES
The
metals that Steelworkers work with are divided into two general
classifications: ferrous and nonferrous. Ferrous metals are those composed
primarily of iron and iron alloys. Nonferrous metals are those composed
primarily of some element or elements other than iron. Nonferrous metals or
alloys sometimes contain a small amount of iron as an alloying element or as an
impurity.
1.FERROUS METALS
Ferrous
metals include all forms of iron and steel alloys. A few examples include
wrought iron, cast iron, carbon steels, alloy steels, and tool steels. Ferrous
metals are iron- base alloys with small percentages of carbon and other elements
added to achieve desirable properties. Normally, ferrous metals are magnetic
and nonferrous metals are nonmagnetic.
IRON
Pure iron
rarely exists outside of the laboratory. Iron is produced by reducing iron ore
to pig iron through the use of a blast furnace. From pig iron many other types
of iron and steel are produced by the addition or deletion of carbon and
alloys. The following paragraphs discuss the different types of iron and steel
that can be made from iron ore.
PIG IRON
Pig iron is composed of about 93% iron, from 3% to
5% carbon, and various amounts of other elements. Pig iron is comparatively
weak and brittle; therefore, it has a limited use and approximately ninety
percent produced is refined to produce steel. Cast-iron pipe and some fittings
and valves are manufactured from pig iron.
WROUGHT IRON
Wrought iron is made from pig iron with some slag
mixed in during manufacture. Almost pure iron; the presence of slag enables
wrought iron to resist corrosion and oxidation. The chemical analyses of
wrought iron and mild steel are just about the same. The difference comes from
the properties controlled during the manufacturing process. Wrought iron can be
gas and arc welded, machined, plated, and easily formed; however, it has a low
hardness and low-fatigue strength.
CAST IRON
Cast iron is any iron containing greater than 2%
carbon alloy. Cast iron has a high- compressive strength and good wear
resistance; however, it lacks ductility, malleability, and impact strength.
Alloying it with nickel, chromium, molybdenum, silicon, or vanadium improves
toughness, tensile strength, and hardness. A malleable cast iron is produced
through a easily as the low-carbon steels. They are used for crane prolonged
annealing process. hooks, axles, shafts, setscrews, and so on.
INGOT IRON
Ingot iron is a commercially pure iron (99.85%
iron) that is easily formed and possesses good ductility and corrosion
resistance. The chemical analysis and properties of this iron and the lowest
carbon steel are practically the same. The lowest carbon steel, known as dead-
soft, has about 0.06% more carbon than ingot iron. In iron the carbon content
is considered an impurity and in steel it is considered an alloying element.
The primary use for ingot iron is for galvanized and enameled sheet.
STEEL
All the different metals and materials that we
use in our trade, steel is by far the most important. When steel was developed,
it revolutionized the American iron industry. With it came skyscrapers,
stronger and longer bridges, and railroad tracks that did not collapse. Steel
is manufactured from pig iron by
decreasing the amount of carbon and other impurities and adding specific
amounts of alloying elements. Do not confuse steel with the two general classes
of iron: cast iron (greater than 2% carbon) and pure iron (less than 0.15% carbon). In steel manufacturing, controlled
amounts of alloying elements are added during the molten stage to produce the
desired composition. The composition of a steel is determined by its
application and the specifications that
were developed by the following: American Society for Testing and Materials
(ASTM), the American Society of Mechanical Engineers (ASME), the Society of
Automotive Engineers (SAE), and the American Iron and Steel Institute
(AISI).Carbon steel is a term applied to a broad range of steel that falls between the
commercially pure ingot
iron and the cast irons. This range of carbon steel may be classified
into four groups:
HIGH-CARBON STEEL/VERY HIGH-CARBON STEEL
Steel in these
classes respond well
to heat treatment
and can be welded. When welding, special electrodes
must be used along with preheating and stress- relieving procedures to prevent
cracks in the weld areas. These steels are
used for dies, cutting
tools, milltools, railroad car
wheels, chisels, knives, and so on.
LOW-ALLOY, HIGH-STRENGTH, TEMPERED STRUCTURAL
STEEL
A special
lowcarbon steel, containing specific small amounts of alloying elements, that
is quenched and tempered to get a yield strength of greater than 50,000
psi and tensile
strengths of 70,000 to
120,000 psi. Structural members
made from these high-strength steels may
have smaller cross- sectional areas than
common structural steels
and still have equal or greater strength. Additionally,
these steels are normally more corrosion- and abrasionresistant.
High-strength
steels are covered by ASTM specifications. NOTE: This type of
steel is much tougher than low-carbon
steels. Shearing machines for this type of steel must have twice the
capacity than that required for low-carbon steels
STAINLESS STEEL
This type
of steel is classified by the American Iron and Steel Institute (AISI) into two
general series named the 200-300 series and 400 series. Each series includes several
types of steel with different characteristics. The 200-300 series of stainless
steel is known as
AUSTENITIC.
This
type of steel
is very tough and ductile in the as-welded condition; therefore, it is ideal for welding and requires no annealing
under normal atmospheric conditions.
The most well-known types of steel
in this series are the 302 and
304. They are commonly called
18-8 because they are composed of 18% chromium and 8% nickel.
The
chromium nickel steels
Low-Carbon
Steel . . . 0.05% to 0.30% carbon are
the most widely used
and are
normally nonmagnetic.
Medium-Carbon
Steel . . . 0.30% to 0.45% carbon
High-Carbon
Steel . . . 0.45% to0.75% carbon their
crystalline structure
into two
general groups.
One Very
High-Carbon Steel . . . 0.75% to 1.70% carbon group is known as
FERRITIC
CHROMIUM and the other group as MARTENSITIC CHROMIUM.
2.ALLOY STEELS
Steels
that derive their properties primarily from the presence of alloying element other
than carbon are called ALLOYS or ALLOY STEELS. Note, however, that alloy steels
always contain traces of other elements. Among the more common alloying
elements are nickel, chromium, vanadium, silicon, and tungsten. One or more of
these elements may be added to the steel during the manufacturing process to
produce the desired characteristics.
Alloy
steels may be produced in structural sections, sheets, plates, and bars for use
in the as rolled condition. Better physical properties are obtained with these
steels than are possible with hot. These alloys are used in structures where
the strength of material is especially important. Bridge members, railroad
cars, dump bodies, dozer blades, and crane booms are made from alloy steel.
Some of the common alloy steels are briefly described in the paragraphs below.
NICKEL STEELS
These
steels contain from 3.5% nickel to 5% nickel. The nickel increases the strength
and toughness of these steels. Nickel steel containing more than 5% nickel has
an increased resistance to corrosion and scale. Nickel steel is used in the
manufacture of aircraft parts, such as propellers and airframe support members.
CHROMIUM STEELS
These
steels have chromium added to improve hardening ability, wear resistance, and
strength. These steels contain between 0.20% to 0.75% chromium and 0.45% carbon
or more. Some of these steels are so highly resistant to wear that they are
used for the races and balls in antifriction bearings.
Chromium
steels are highly resistant to corrosion and to scale.
CHROME VANADIUM STEEL
This steel has the maximum amount of strength with
the least amount of weight. Steels of this type contain from 0.15% to 0.25%
vanadium, 0.6% to 1.5% chromium, and 0.1% to 0.6% carbon. Common uses are for
crankshafts, gears, axles, and other items that require high strength. This
steel is also used in the manufacture of high-quality hand tools, such as
wrenches and sockets.
TUNGSTEN STEEL
This is a special alloy that has the property of
red hardness. This is the ability to continue to cut after it becomes red-hot.
A good grade of this steel contains from 13% to 19% tungsten, 1% to 2%
vanadium, 3% to 5% chromium, and 0.6% to 0.8% carbon. Because this alloy is
expensive to produce, its use is largely restricted to the manufacture of drills,
lathe tools, milling cutters, and similar cutting tools.
MOLYBDENUM
This is
often used as an alloying agent for steel in combination with chromium and
nickel. The molybdenum adds toughness to the steel. It can be used in place of
tungsten to make the cheaper grades of high-speed steel and in carbon
molybdenum high-pressure tubing.
MANGANESE STEELS
The amount of manganese used depends upon the
properties desired in the finished product. Small amounts of manganese produce
strong, free-achgining steels. Larger amounts (between 2% and 10%) produce a
somewhat brittle steel, while still larger amounts (11% to 14%) produce a steel
that is tough and very resistant to wear after proper heat treatment.
3. NONFERROUS METALS
Nonferrous
metals contain either no iron or only insignificant amounts used as an
alloy.
Some of the more common nonferrous metals
Steelworkers work with are as follows: copper, brass, bronze, copper-nickel
alloys, lead, zinc, tin, aluminum, and Duralumin. NOTE: These metals are nonmagnetic.
COPPER
This
metal and its alloys have many desirable properties. Among the commercial
metals, it is one of the most popular. Copper is ductile, malleable, hard,
tough, strong, wear resistant, machinable, weld able, and corrosion resistant.
It also has high-tensile strength, fatigue strength, and thermal and electrical
conductivity. Copper is one of the easier metals to work with but be careful
because it easily becomes work-hardened; however, this condition can be
remedied by heating it to a cherry red and then letting it cool. This process,
called annealing, restores it to a softened condition. Annealing and softening
are the only heat-treating procedures that apply to copper. Seams in copper are
joined by riveting, silver brazing, bronze brazing, soft soldering, gas
welding, or electrical arc welding. Copper is frequently used to give a
protective coating to sheets and rods and to make ball floats, containers, and
soldering coppers.
CARBON STEELS
Carbon
steels are iron-carbon alloys containing up to 2.06% of carbon, up to1.65% of
manganese, up to 0.5% of silicon and sulfur and phosphorus as impurities.
Carbon content in carbon steel determines its strength and ductility. The
higher carbon content, the higher steel strength and the lower its ductility.
According to the steels classification there are following groups of carbon
steels:
• Low
carbon steels (C < 0.25%)
• Medium
carbon steels (C =0.25% to 0.55%)
• High
carbon steels (C > 0.55%)
• Tool
carbon steels (C>0.8%)
Designation
system of carbon steels Chemical compositions of some carbon steels Properties
of some carbon steels
Low carbon steels (C < 0.25%)
Properties:
good formability and weldability, low strength, low cost.
Applications:
deep drawing parts, chain, pipe, wire, nails, some machine parts.
Medium carbon steels (C =0.25% to 0.55%)
Properties:
good toughness and ductility, relatively good strength, may be hardened by
quenching
Applications:
rolls, axles, screws, cylinders, crankshafts, heat treated machine parts.
High carbon steels (C > 0.55%)
Properties:
high strength, hardness and wear resistance, moderate ductility.
Applications:
rolling mills, rope wire, screw drivers, hammers, wrenches, band saws.
Tool carbon steels (C>0.8%) -
subgroup of high carbon steels
Properties:
very high strength, hardness and wear resistance, poor weldability, low
ductility.
Applications:
punches, shear blades, springs, milling cutters, knives, razors. Designation
system of
carbon steels
American
Iron and Steel Institute (AISI) together with Society of Automotive Engineers
(SAE)
have established four-digit (with additional letter prefixes) designation
system:
LOW-ALLOY, HIGH-STRENGTH, TEMPERED STRUCTURAL STEEL
A special
lowcarbon steel, containing specific small amounts of alloying elements, that
is quenched and tempered to get a yield strength of greater than 50,000 psi and
tensile strengths of 70,000 to 120,000 psi. Structural members made from these
high-strength steels may have smaller cross- sectional areas than common
structural steels and still have equal or greater strength. Additionally, these
steels are normally more corrosion- and abrasionresistant. High-strength steels
are covered by ASTM specifications. NOTE: This type of steel is much tougher
than low-carbon steels. Shearing machines for this type of steel must have
twice the capacity than that required for low-carbon steels
STAINLESS STEEL
This type
of steel is classified by the American Iron and Steel Institute (AISI) into two
general series named the 200-300 series and 400 series. Each series includes
several types of steel with different characteristics. The 200-300 series of
stainless steel is known as austenitic.
AUSTENITIC
This type
of steel is very tough and ductile in the as-welded condition; therefore, it is
ideal for welding and requires no annealing under normal atmospheric
conditions. The most well-known types of steel in this series are the 302 and
304. They are commonly called 18-8 because they are composed of 18% chromium
and 8% nickel. The chromium nickel steels Low-Carbon SAE 1XXX
First
digit 1 indicates carbon steel (2-9 are used for alloy steels); Second digit
indicates modification of the steel.
0 - Plain
carbon, non-modified
1 -
Resulfurized
2 -
Resulfurized and rephosphorized
5 -
Non-resulfurized, Mn over 1.0%
Last two
digits indicate carbon concentration in 0.01%.
Example:
SAE 1030 means non modified carbon steel, containing 0.30% of carbon.
A letter
prefix before the four-digit number indicates the steel making technology:
A - Alloy, basic open hearth
B - Carbon, acid
Bessemer
C - Carbon, basic open hearth
D - Carbon, acid open hearth
E -
Electric furnace
Example:
AISI B1020 means non modified carbon steel, produced in acid Bessemer and
containing 0.20% of carbon.
Chemical
compositions of some carbon steels
SAE/AISI
grade C, % Mn,% P,% max S,% max
1006 0.08 max 0.35 max 0.04 0.05
1010 0.08-0.13 0.30-0.60 0.04 0.05
1020 0.17-0.23 0.30-0.60 0.04 0.05
1030 0.27-0.34 0.60-0.90 0.04 0.05
1045 0.42-0.50 0.60-0.90 0.04 0.05
1070 0.65-0.76 0.60-0.90 0.04 0.05
1090 0.85-0.98 0.60-0.90 0.04 0.05
1117 0.14-0.20 1.10-1.30 0.04 0.08-0.13
1547 0.43-0.51 1.35-1.65 0.04 0.05
Related Topics