Chapter: Chemistry : Electro Chemistry and Corrosion
Corrosion
1 Corrosion
1.1 Consequences of corrosion
2 Types of Theories of Corrosion
2.1 Dry or Chemical corrosion
2.2 Wet or electrochemical corrosion
3 Factors influencing the rate of corrosion
3.1 Nature of the metal
3.2 Nature of the environment
4 Corrosion Control
4.1 By modifying metal
4.2 BY modifying the environment
5 Paints
5.1 Characteristics of a good paint
5.2 Constituents and their functions of paints
6 Metallic Coatings
6.1 Electroplating
6.2 Electroless Plating
1 Corrosion
It is the gradual deterioration of metals by
chemical, electrochemical or biochemical interaction with the environment.
Causes of Corrosion
Metals occur in nature as their oxides, sulphides
carbonates etc. The chemically combined state is thermodynamically more stable.
When we extract a metal from its ore, the metal is in a higher energy state,
which is thermodynamically unstable. So it tries to go back to the stable state
by chemical or electrochemical interaction with the environment.
Consequences
or effects of Corrosion
Efficiency of the machine decreases.
Plant has to be shut down due to failure.
Product is contaminated.
The toxic products of corrosion cause health
hazards.
There is a necessity to over design to allow for
corrosion.
2 Types or
Theories of Corrosion
I. Dry or Chemical Corrosion
II. Wet
or Electrochemical Corrosion
2.I. Dry or Chemical Corrosion
It is due
to the attack on metal surface by atmospheric gases like O2, SO2, H2S etc.
(e.g.) Tarnishing of silver by H2S.
There are
three types of dry or chemical corrosion.
Oxidation Corrosion
Corrosion by Hydrogen
Liquid Metal Corrosion
Oxidation Corrosion
It is due
to the direct attack of oxygen on metal surface in the absence of moisture.
Alkali and Alkaline earth metals are corroded at low temperatures. At high
temperatures, most metals except Au, Pt and Ag are oxidized.
Mechanism
Oxidation occurs at the surface of the metal to
form M2+ ions.
® M2+
+ 2e-
Oxygen takes up the electrons. O2 is reduced to O2-
O2 + 2e-
® O2-
O2- ion reacts with M2+ to
form metal oxide.
M2+
+ O2- ® MO
The metal
surface is converted to a monolayer of
metal oxide. Further corrosion occurs by diffusion of M2+ ion through
the metal oxide barrier. The growth of oxide film is perpendicular to the metal
surface.
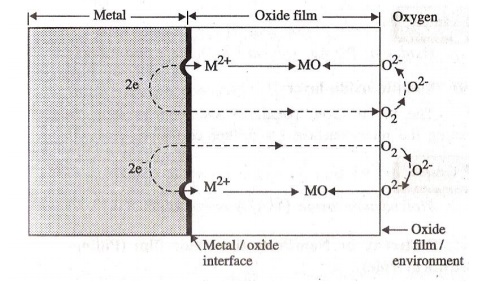
Different types of oxide films are formed.
(i) Porous and Non-Porous Oxide Film (or)
Pilling-Bedworth Rule
(a) If
the volume of the oxide layer formed is less than the volume of the consumed,
the oxide layer is porous. (e.g.) The volumes of oxides of alkali and alkaline
earth metals are less than the volume of the metal consumed. So the oxide layer
is porous and non-protective
(b) If
the volume of the oxide layer formed is greater than the volume of the metal
consumed, the oxide layer is non-porous.(e.g.) The volumes of oxides of heavy
metals such as Pb, Sn are greater than the volumes of the metal consumed. So
the oxide layer is non-porous and protective.
(ii) Stable Oxide Layer
A stable
oxide layer is firmly adsorbed on the metal surface. The layer is impervious
and prevents further corrosion. So the layer itself acts as a protective
coating. (E.g.) Oxides of Al, Cu etc.
(iii) Unstable oxide Layer
This is
mainly produced on the surface of noble metals such Ag, Au etc. The unstable
oxide decomposes to stable metal and oxygen. Metal Oxide Metal + Oxygen
(iv) Volatile Oxide
The oxide
film volatilizes as soon as it is formed. It leaves fresh metal surface for
further continuous attack. (e.g.) Molybdenum oxide MoO3.
(2) Corrosion by Hydrogen
Hydrogen embrittlement Definition
It is
formation of cracks and blisters on the metal by hydrogen gas when the metal
comes into contact with H2S. Iron liberates atomic hydrogen by reacting with
H2S.
Fe +
H2S FeS + 2H
Hydrogen
atoms diffuse into the voids of metal matrix. When the pressure of the gas
increases, cracks and blisters develop on the metal.
Decarburisation
It is the
process of decrease in the carbon content of steel. At high temperature,
molecular hydrogen decomposes to atomic hydrogen. High Temperature
When steel is exposed to this environment, carbon in the steel reacts with atomic hydrogen.
C + 4H - - > CH4
Hence the
carbon content in steel decreases. Collection of methane gas in the voids of
steel develops high pressure and causes cracking.
(3) Liquid Metal Corrosion
It is due
to the chemical action of flowing liquid metal at high temperature. It involves
:
dissolution of a solid metal by the liquid metal.
Penetration of liquid metal into the solid metal.
2. 2. Wet (or) Electrochemical
Corrosion :
It occurs
under the following conditions.
When two
dissimilar metals or alloys are in contact with each other in presence of an
aqueous solution or moisture.
When the metal is exposed to an electrolyte with
varying amounts of oxygen.
Mechanism of Wet Corrosion
Metal dissolution occurs at the anode.
M → Mn++
+ ne-
Reduction reaction occurs at the cathode in
different environments.
Acidic
environment : Here hydrogen gas is evolved at the cathode.
2 H+ +
2e- → H2
(b)
Neutral environment : In neutral or slightly alkaline medium, hydroxide ions
are formed at the cathode.
½ O2 +
2e- + H2O → 2OH-
(a) Hydrogen Evolution type
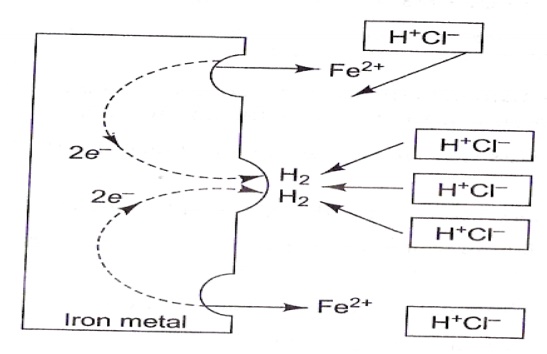
corrosion (In Acidic Medium)
All
metals above hydrogen in the electrochemical series tend to get dissolved
in acidic
solution with simultaneous evolution of H2 gas. e.g.) When iron comes into
contact with non-oxidising acid like HCl, hydrogen evolution occurs.
At anode
: Iron is oxidized to Fe2+
Fe → Fe+2
+ 2e-
At
cathode : H+ ion is reduced to H2.
2 H+ +
2e- → H2
oxygen,
OH- ions are formed.
At anode
: Iron is oxidized to Fe
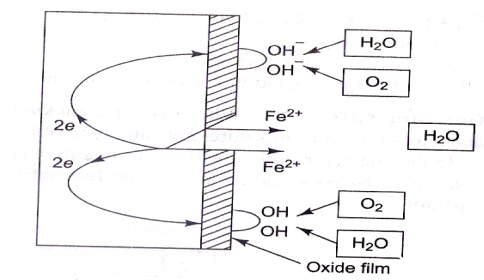
(b) Absorption of Oxygen (or)
Formation of hydroxide ion type corrosion (In neutral or weakly alkaline
medium)
The
surface of iron is normally coated with a thin film of iron oxide. But if some
cracks develop on the film, anodic areas are created on the surface. The rest
of the metal part acts as cathode.(e.g.) When iron is in contact with an electrolyte
solution in presence ofoxygen, OH- ions are formed. At anode : Iron is oxidized
to Fe+2
At
cathode : Production of OH- ions (more aeration) ½ O2 + 2e- + H2O → 2OH
Waterline corrosion
Let us
consider metal tank partially filled up with water. The metal area above water
line is exposed to higher concentration of oxygen (cathode) than the metal
below water level. The metal less exposed to O2 acts as anode and corrodes.
This is called water line corrosion.
Examples of differential aeration
corrosion
Pitting
or localized corrosion
Crevice corrosion
Pipeline corrosion
iv)
Corrosion on wire fence
(i) Pitting Corrosion
It is the
localized attack resulting in the formation of a hole due to corrosion. Example
: Metal area covered by a drop of water, sand, dirt etc.
The area
covered by the drop or dirt acts as anode and corrodes. Theuncovered area
exposed to air or O2 acts as cathode.The rate of corrosion is more if the
cathodic area is larger and anodic area is smaller. Thus more material is
removed from the same area and a pit is formed.
At anode
: Iron is oxidized to Fe+
Fe → Fe2+
+ 2e-
At
cathode : O2 is reduced to OH-. ½ O2 + H2O + 2e- → 2OH Overallreaction :
Fe2+ +
OH- → Fe(OH)
At anode
: Iron is oxidized to Fe+
Fe → Fe2+
+ 2e-
At
cathode : O2 is reduced to OH-. ½ O2 + 2e- + H2O → 2OH
Overall Reaction
Fe+2 +
2OH- → Fe(OH)
If enough
oxygen is present, Fe(OH)2 is oxidized to Fe(OH)2.
4Fe(OH)2 + O2 + H2O → 4Fe(OH)
Differences between chemical
corrosion and electro-chemical corrosion: Chemical Corrosion Electro-chemical
Corrosion
It occurs in dry condition It occurs in presence of
moisture or electrolyte.
It occurs
by the direct chemical attack on the metal by the environment.It occurs by the
formation of a large number of anodic and cathodic areas.
Even a
homogenous metal surface is corroded. Only heterogeneous or bimetallicsurface is
corroded.
Corrosion
products gather at the place of corrosion. Corrosion occurs at the anode, while
the products form elsewhere.
It is a self controlled process It is a continuous
process
It takes
place by adsorption mechanism. It follows electrochemical reaction.(e.g.) Mild
scale formation on iron surface (e.g.) Rusting of iron under moist atmosphere
Types of electrochemical
corrosion
There are
two types:
(i)
Galvanic corrosion
(ii)
Differential aeration or Concentration cell corrosion
(i) Galvanic corrosion
When two
different metals are in contact with each other in presence of aqueous solution
or moisture, galvanic corrosion takes place.
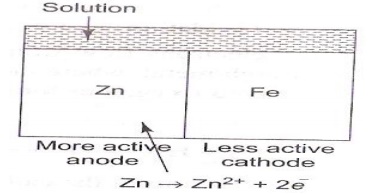
The metal
with more negative electrode potential acts as anode. Metal with less negative
electrode potential acts as cathode. In the Zn-Fe couple as shown in the
figure, zinc with more negative electrode potential, dissolves in preference to
iron. Zn acts as anode and Fe as cathode.
Example :
Steel
screw in a brass marine hardware easily undergoes corrosion. Iron has E0 =
-0.44V. For Cu E0 = +0.34 V. Iron corrodes in preference to Cu.
Prevention
Galvanic
corrosion is minimized by providing an insulation between the two metals.
(ii) Differential aeration (or)
concentration cell corrosion
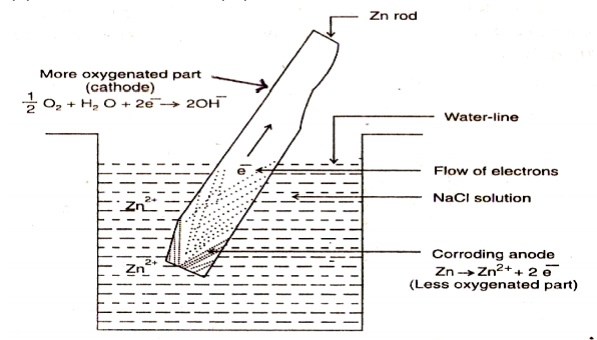
Let a metal be partially immersed in a conducting solution. The part of the metal above the solution is more aerated and acts like cathode. The less aerated metal part inside the solution acts as anode and corrodes.
At anode
: Corrosion occurs (less aeration)
M → M2+ +
2e
At
cathode : Production of OH- ions (more aeration) ½ O2 + 2e- + H2O → 2OH
Wateline corrosion
Let us
consider metal tank partially filled up with water. The metal area above water
line is exposed to higher concentration of oxygen (cathode) than the metal
below water level. The metal less exposed to O2 acts as anode and corrodes.
This is called water line corrosion.
Examples of differential aeration
corrosion
Pitting
or localized corrosion
Crevice corrosion
Pipeline corrosion
Corrosion on wire fence
(i) Pitting Corrosion
It is the
localized attack resulting in the formation of a hole due to corrosion. Example
: Metal area covered by a drop of water, sand, dirt etc.
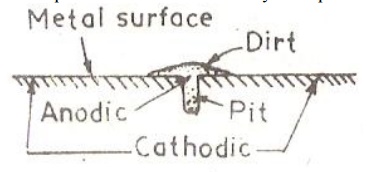
The area
covered by the drop or dirt acts as anode and corrodes. The uncovered area
exposed to air or O2 acts as cathode. The rate of corrosion is more if the
cathodic area is larger and anodic area is smaller. Thus more material is
removed from the same area and a pit is formed.
At anode
: Iron is oxidized to Fe+2
Fe → Fe2+
+ 2e-
At
cathode : O2 is reduced to OH-. ½ O2 + H2O + 2e- → 2OH Overall reaction :
Fe2+ +
OH-→ Fe(OH)
(ii) Crevice Corrosion
Let a
crevice or crack between two different metallic objects be in contact
with a
liquid. The crevice acts like anode due to less oxygen availability and
corrodes. The exposed area acts as cathode.
(e.g.)
rivets, joints.
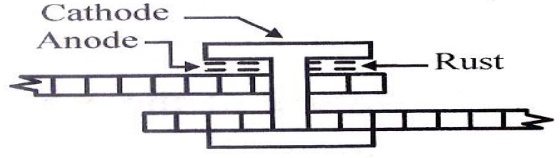
(iii) Pipeline Corrosion
Buried
pipelines or cables passing from one type of soil (clay, less aerated) to
another type (sand, more aerated) get corroded due to differential aeration.
(iv) Corrosion on wire fence
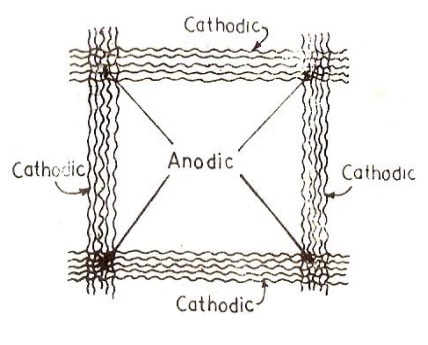
In a wire
fence, the wires at the crossings are less aerated than the rest of the
fence. So
corrosion takes place at the wire crossings, which become anodic.
3
Factors
influencing corrosion
3.1 Nature of the metal
(i) Position in emf series
Metals
above hydrogen in the electrochemical series corrode easily because they have
negative reduction potential. When two metals are in contact, the more active metal
with a higher negative potential corrodes.
(ii) Areas of anode and cathode
Corrosion
will be severe if the anodic area is smaller and cathodic area is larger. The
larger cathodic area demands more electrons. So the anodic area corrodes
faster.
(iii) Purity
100% pure
metal will not corrode. (e.g.) Pure Zn does not corrode. If the metal has trace
amount of impurity, it corrodes. (e.g.) Zinc metal with iron or copper impurity
forms an electrochemical cell. The base metal Zn acts as anode and corrodes.
(iv) Over Voltage
Corrosion
rate is inversely proportional to the over voltage of the metal in a corrosive
surroundings. (e.g.) The hydrogen over voltage of Zn in 1M H2SO4 is 0.7V. So
the rate of corrosion is low. But when some Cu impurity is present, the over
voltage is reduced and corrosion rate increases.
(v) Nature of the Film
Nature of
film formed on the metal surface determines extent of corrosion.(e.g.) In the
case of alkali and alkaline earth metals, the oxide film formed is porous .The
corrosion continues. In the case of heavy metals, the oxide film is non-porous.
The film acts as a protective layer.
(vi) Nature of corrosion product
If the
corrosion product is soluble in the corroding medium, corrosion rate is faster.
Similarly if the corrosion product is volatile (e.g. MoO3), corrosion will be
more.
3.2 Nature of Environment
(i) Temperature
Increase
of temperature increases corrosion rate because the rate of diffusion of ions
increases.
(ii) Humidity
Rate of
corrosion is more, if humidity of environment is high. Moisture acts as solvent
for O2, CO2 etc, to produce electrolyte necessary for formation of corrosion
cell.
(iii) Corrosive gases
Acidic
gases like CO2, SO2, H2S etc, produce electrolytes and increase corrosion.
(iv) Presence of suspended
particles
Particles
like NaCl, (NH4)2SO4 along with moisture are powerful electrolytes and increase
rate of corrosion.
(v) Effect of pH
Generally
in alkaline medium, the rate of corrosion is less compared to acidic medium.
The
effect of pH on the corrosion of iron in water is shown in the Pourbaix diagram
as indicated in the figure.
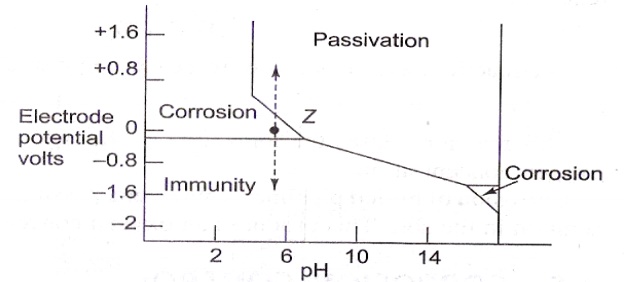
The
figure shows zones of corrosion, immunity and passivity. Z is the point at
which pH=7 and corresponding electrode potential is E= -0.2V. This is in the
corrosion zone. So iron rusts under these conditions.
The rate
of corrosion can be altered by shifting the point Z to different regions.
If the
potential is changed to -0.8V by applying external current, iron becomes immune
to corrosion.
If the potential applied is positive, iron becomes
passive.
If the pH is increased to more than 7, corrosion
rate decreases.
If the pH is reduced to less than 7, rate of
corrosion increases
4 CORROSION CONTROL
The rate
of corrosion can be controlled by modifying the metal or environment. Some
control methods are
proper selection of metals
Use of pure metals
Use of metal alloys
Cathodic protection
Sacrificial anode protection
Impressed
current cathodic protection 5) Changing the environment
6) Use of
inhibitors
Anodic inhibitors
Cathodic inhibitors
7)
Applying protective coatings
1) Proper selection of metals
Noble
metals are used in ornaments and in surgical instruments, because they do not
corrode. Contact of dissimilar metals far away from each other in
electrochemical series should be avoided.
2) By using pure metals
Pure
metals have high corrosion resistance. Even a trace of impurity will lead to
corrosion, the base metal becoming anode.
3) Use of alloys
Use of
metal alloys is a good method of protection against corrosion. (e.g.) Stainless
steel containing chromium forms a coherent oxide film which protects steel
against further attack
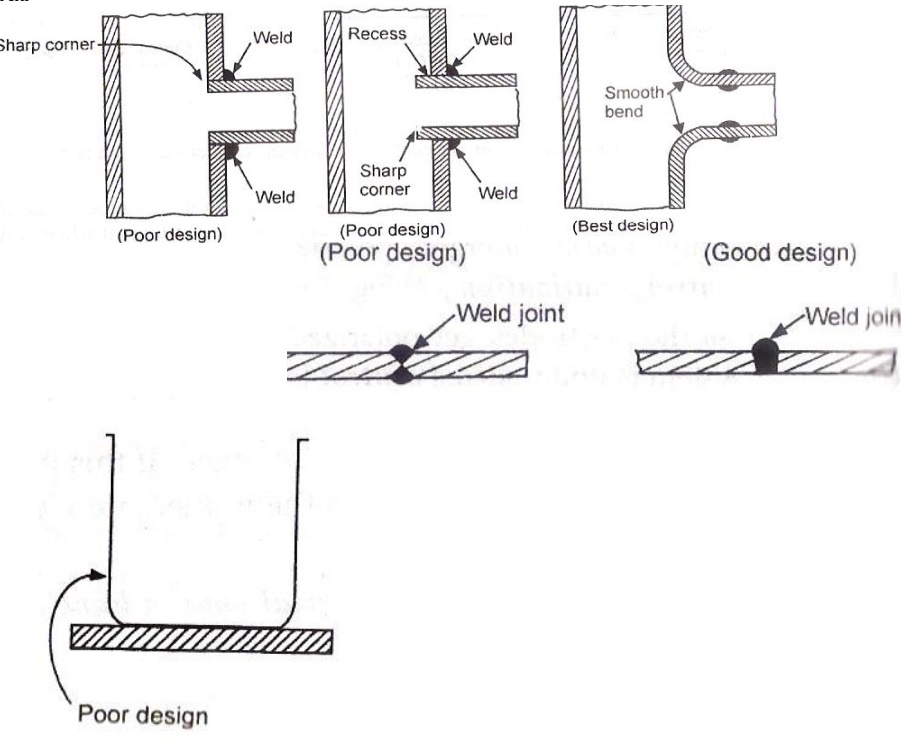
4.1 Proper designing
Complicated
designs with more angles, sharp edges and corners should be avoided.
Direct
contact of dissimilar metals lead to galvanic corrosion. So insulating material
between the two metals should be inserted.
Smaller area for cathode and larger area for anode
must be provided.
Tanks and containers should be designed such that
the liquid should be drained off completely.
Crevices should be avoided or they should be filled
using fillers.
Bendings should be smooth.
Annealing
minimizes corrosion.
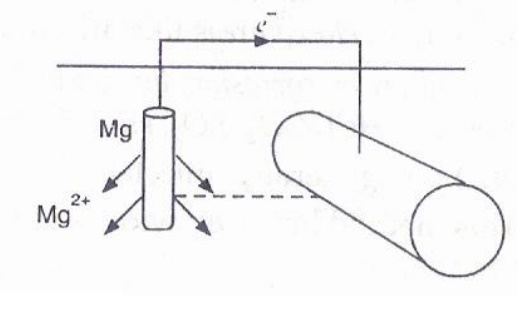
5) Cathodic Protection
The metal
to be protected is made to act like a cathode. This is achieved in two ways.
a) Sacrificial anodic protection
Here the
metal to be protected is made cathode by connecting it to a more active metal
(anodic metal) called sacrificial anode. Only the more active metal will
a) Sacrificial anodic protection
Here the
metal to be protected is made cathode by connecting it to a more active metal
(anodic metal) called sacrificial anode. Only the more active metal will be
corroded, protecting the parent metal. Since the anodic metal is sacrificed,
the method is called sacrificial anodic protection. Mg, Zn are used as
sacrificial anodes. Metal to be protected
Applications
Protection
of buried pipelines, cables
Protection of ships and boats
Calcium metal is used to minimize engine corrosion
Magnesium
sheets are inserted into domestic water boilers to prevent rust formation.
b) Impressed current cathodic
protection method
Here an
impressed current is applied in an opposite direction to annul the corrosion
current. Thus the corroding metal is converted to cathode from anode.The
negative terminal of battery is connected to the metal to be protected. The
positive terminal is connected to an inert electrode like graphite. The anode
is buried in a „back-fill‟ (containing a mixture of gypsum, coke breeze and
sodium sulphate) to increase electrical contact.
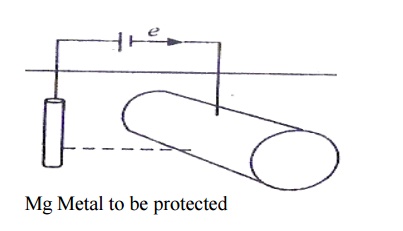
Mg Metal
to be protected
APPLICATION
Protection
of tanks, transmission line towers, underground water pipes, oil pipe line,
ships etc.
Limitations
It is
costly
It fails when current is switched off.
Corrosion inhibitors
A
corrosion inhibitor is a substance that reduces corrosion, when added to the
corrosive environment. There are three types of inhibitors.
Anodic
inhibitors - chromate, nitrate
Cathodic inhibitors - amines
Vapour phase inhibitors - benzonitrile.
Anodic
inhibitors
(e.g.)
chromate, nitrate, phosphates, tungstate.
The
inhibitors form insoluble compound with the newly produced metal ions and
prevent corrosion. This compound is adsorbed on the metal surface to form a
passive film. Anodic inhibitors are used to repair
i) the
crack of oxide film on metal surface
pitting corrosion
porous oxide film on metal surface
Cathodic
inhibitors
There are
two types depending on the nature of cathodic reaction in an electrochemical
reaction.
a) In acidic solution
Example :
amines, thiourea, mercaptans act as inhibitors. Here evolution of H2 is the
cathodic reaction.
2 H+ +
2e- → H2
The
corrosion is controlled by slowing down the diffusion of H+ ions to cathode by
addition of the inhibitor which is adsorbed on the metal surface.
b) In neutral solution
Example :
hydrazine, sodium sulphite act as inhibitors. Here OH- ions are formed at
cathode. H2O + ½ O2 + 2e- → 2 OH
Corrosion
is controlled by eliminating O2 from the corroding medium by adding Na2SO3. The
OH- ions can be eliminated by salts of Mg, Zn etc.
iii) Vapour phase inhibitors
(e.g.)
benzotriazole, dicyclohexyl ammonium chromate act as inhibitors. These organic
inhibitors readily vapourise and form a protective layer on the metal surface.
4.2 Control of corrosion by
modifying the environment:
There are
five methods
1. Deaeration :
Presence
of oxygen increases corrosion rate. Deaeration involves removal of dissolved
oxygen by increasing the temperature together with the mechanicalagitation.
This also removes dissolved oxygen.
2. Deactivation:
It is the
removal of dissolved oxygen by adding chemicals in aqueous solution.
(E.g.)
2Na2SO3 + O2 → 2Na2SO4
3. Dehumidification:
It is the
removal of moisture from the air by reducing the relative humidity of the
surrounding air. It can be achieved by adding silica gel or alumina which
absorbs
moisture.
4. Alkaline neutralization:
The
acidic nature of the corrosive environment is due to the presence of HCl, SO2,
CO2 etc. They are neutralized with alkali spray. E.g. NaOH lime etc.
5. Using corrosion inhibitors :
A
corrosion inhibitor is a substance that reduces the corrosion of a metal when
added to corrosive environment
Applications
To
prevent corrosion in closed space, storage containers, sophisticated equipment
etc.
PROTECTIVE COATINGS
Metal
surface is covered by a protective coating to prevent corrosion. The coating
acts as a physical barrier between the metal surface and the environment. The
coating gives a decorative appeal and also imparts hardness, oxidation
resistance and thermal insulation to the surface. The main types of coating
are:
Metallic coating
Chemical conversion coating
Organic coating
Non-metallic coating
5 PAINT
Paint is
a mechanical dispersion of one or more fine pigments in a medium (thinner +
vehicle). When a paint is applied to metal surface, the thinner evaporates. The
vehicle undergoes slow oxidation to form a pigmented film.
5.1 Requirements or requisites of
a good paint
A good
paint should,
have good
covering power
spread easily on the surface
not crack on drying
adhere well to the surface
give a glossy film
be corrosion and water resistant
have stable colour
5.2Constituents of Paint
Pigment
Vehicle Thinner Drier Filler Plasticizer
Anti
skinning agent
1. Pigment
It is a
solid that gives colour to the paint.
Functions:
1. To
give colour and opacity to the film.
2. To
provide strength to the film.
3. To
protect film by reflecting U.V. rays.
4. To
provide resistance to abrasion and weather.
Example:
White
pigment - White lead, TiO2
Blue
pigment - Prussion blue
Green
pigment - Chromium oxide
Red
pigment - Red lead, Fe3O4
2. Vehicle (or) Drying Oil
It is the
film-forming liquid. It holds the ingredients of the paint. It is a nonvolatile
high molecular weight fatty acid of vegetable or animal.
Function
1. To
hold the pigment on the surface.
2. To
form a protective layer by oxidation and polymerization. 3. To impart water repellency, toughness and
durability of film. 4. To improve adhesion of film.
Example
Lin seed
oil, Castor oil.
3. Thinner
It is the
volatile portion of paint. It is added to reduce the viscosity of the paintfor
easy application on the surface. It easily evaporates after paint is applied.
Functions
1. To
reduce viscosity of paint.
2. To
dissolve vehicle and other additives.
3. To
suspend the pigments.
4. To
increase elasticity of film.
5. To
increase penetration of vehicle.
6. To
improve drying of film.
Example
Turpentine,
Dipentine, Xylol.
4. Drier
It is a
substance used to speed up drying of the paint.
Functions
1. To act
as oxygen carrier or catalys.
2.To
provide oxygen essential for oxidation and polymerization of drying oil.
Example
Metallic
soap, linoleate and resinate of Co, Mn etc.
5. Extender or Filler
These are
white pigments that form bulk of the paint.
Functions
1. To
reduce cost of paint
2. To
prevent shrinkage and cracking of film
3. To
modify shades of pigment
4. To
retard settling of pigments in paint.
Example
Talc
gypsum, china-day.
6. Plasticizer
It is
added to the paint to provide elasticity to the film and prevent its cracking.
Example
Triphenyl
phosphate, Tricresyl phosphate
7. Antiskinning agent
It is a
chemical added to the paint to prevent gelling and peeling of the paint.
Example
Polyhydroxy
phenols.
Pigment Volume Concentration
(P.V.C.)
The
P.V.C. of a paint is calculated using the equation. P.V.C. =
Volume of
pigment in the paint =Volume of pigment in the paint + Volume of non-volatile
vehicle in the paint
If P.V.C.
is high, durability, adhesion and consistency of the paint will be low.
Failure of Paints
A paint
may fail due to any one of the following reasons:
Chalking : It is the gradual powdering of
the paint film on the painted surface. This happens due to improper dispersion of pigment in vehicle.
Cracking
: A paint
film cracks due to unequal expansion or contraction of paint coats.
Evasion : This is very
quick chalking.
iv) Blistering : It is due to improper
surface exposure of paint to strong sunshine.
6 Metallic Coating
6.1 Electroplating or
Electro-deposition
It is the
deposition of coat metal on the base metal by passing direct current through an
electrolytic solution of a soluble salt of the coat metal. The base metal to be
electroplated acts as cathode. The coat metal or an inert electrode forms
anode. The electrolyte is a soluble salt of coat metal.
Objectives or uses or
applications of Electroplating:
To
enhance resistance to corrosion of base metals.
To give a decorative appearance.
To enhance resistance to chemical attack.
To improve hardness and wearing resistance.
To obtain polished surface.
Theory
If the
coating metal itself forms the anode, the concentration of electrolyte bath
does not change during electrolysis. The metal ions deposited on the cathode
are replenished continuously by dissolution of the anode.
Example
Electroplating of Gold
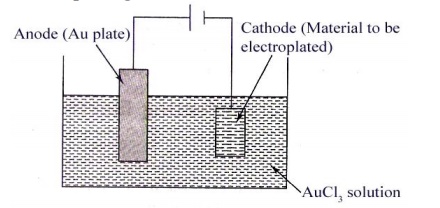
The
object to be gold plated is treated with organic solvent like acetone, CCl
to remove
grease, oil etc. It is then washed with dil H2SO4 to remove scales, oxides etc.
The cleaned object is made cathode of electrolytic cell. Anode is a gold plate.
AuCl3 solution is the electrolyte. When current is passed into the solution,
gold ions migrate to the cathode, get reduced and deposit on the object.

Conditions
Temperature
: 600C
Current density : 1 to 10 mA/cm
Low metal ion concentration
Buffer solution to maintain pH.
Applications or Uses or
Objectives
To give a
decorative appearance.
Electrical and electronic applications
To get a thin coating of gold on cheap jewellery
To achieve oxidation resistance, corrosion
resistance etc.
6.2 Electroless Plating
It is the
deposition of a noble metal (from its salt solution) on a catalytically active
metal surface using a reducing agent without use of electric current.The
reducing agent reduces the metal ions. The metal atoms get deposited over the
surface to give a thin uniform coating.
Metal
ions + reducing agent metal (deposited)
+ oxidation product
Example
Electroless nickel plating
The
various steps are:
Step I : Pretreatment and
activation of the surface:
The
surface to be plated is degreased by using organic solvents or alkali and then
accompanied by acid treatment.
The
surface of stainless steel is activated by dipping in hot solution of 50%
H2SO4.
Mg alloy surface is activated by giving a thin
coating of zinc and copper overit.
Al, Cu, Fe, brass etc, do not require activation.
Plastic, glass etc, are activated by dipping in a
solution of SnCl2/HCl and then
in PdCl2
solution. On drying a thin layer of palladium is formed on the surface.
Step II : Preparation of plating
bath:
The
plating bath consists of:
Coating
Metal : A solution of NiCl2 20g/lit.
Reducing agent : Sodium hypophosphite 20g/lit.
Exaltant to accelerate coating rate and complexing
agent : Sodium succinate 15g/lit.
Buffer to maintain pH at 4.5 : Sodium acetate
10g/lit.
Temperature 93oC
The
pretreated object is immersed in the plating bath for required time. The
following reactions occur and nickel is coated on the object.
Cathode :
Ni2+ + 2e- → Ni
Anode :
H2PO2- + H2O → H2PO2- + 2H+ + 2e
Overall
Reaction : Ni2+ + H2PO2- + H2O → Ni + H2PO3- + 2H+
Uses of Nickel Plating
For
decorative coating of jewellery, decorative items and automobile spares
For coating of polymers for decorative purpose.
For electronic appliances.
Advantages of electroless plating
over electro plating
Electricity
is not necessary
Complicated parts are uniformly coated
Plastics, glass etc, are easily coated
Good mechanical, chemical and magnetic properties
are obtained.
Differences between
Electroplating and Electroless plating:
Electroplating Electroless
Plating
It is done by passing current. It is done by auto
catalytic redox reaction.
Separate anode is required. Catalytic surface of
the object acts as anode.
Anode reaction: M → Mn+ + ne- Anode reaction : R →
O + ne-
Cathode reaction: Mn+ + ne-→ M Cathode reaction: Mn+ + ne- → M
Irregular objects are not satisfactorily plated All
objects are satisfactorily plated.
Object to be coated forms the cathode .Object to be
coated forms catalytically active surface.
It is carried out on conducting materials.It is
carried out even on insulators.
Related Topics